Regulatory T cells integrate native and CAR-mediated co-stimulatory signals for control of allograft rejection
Isaac Rosado Sanchez1,2, Manjurul Haque1,3, Kevin Salim1,3, Madeleine Speck1,3, Vivian Fung1,3, Dominic Boardman1,3, Majid Mojibian1,3, Giorgio Raimondi4, Megan K Levings1,2,3.
1BC Children’s Hospital Research Institute, Vancouver, BC, Canada; 2School of Biomedical Engineering, University of British Columbia, Vancouver, BC, Canada; 3Department of Surgery, University of British Columbia, Vancover, BC, Canada; 4Department of Plastic and Reconstructive Surgery, Johns Hopkins University School of Medicine, Baltimore, MD, United States
Introduction: Regulatory T cells (Tregs) expressing chimeric antigen receptors (CAR) are a promising tool to promote transplant tolerance. The relationship between CAR structure and Treg function was previously studied in xenogeneic, immunodeficient mice, revealing advantages of CD28-encoding CARs. However, these models could underrepresent interactions between CAR-Tregs, antigen-presenting cells (APCs) and donor-specific antibodies (DSAs). Here we aimed to re-visit the question of optimal CAR design for Tregs in an immunocompetent mouse model of skin transplantation.
Methods: We generated mouse Tregs expressing HLA-A2-specific CARs encoding CD3ζ, with or without different costimulatory domains, and compared their function in vitro and in vivo. In vitro proliferation and cytokine production was tested by co-culture with an HLA-A2+ cell line. In vitro CAR-Treg function was determined using an antigen-dependent linked suppression assay. In vivo function was determined using an HLA-A2+ skin transplant model and measurements of anti-HLA-A2 DSAs in plasma. Co-cultures with CD86+ or CD86- HLA-A2+ cells, and dendritic cell-based suppression assays assessed the contribution of exogenous co-stimulation to CAR-Treg activation and function.
Results: In vitro assays revealed the CD28-encoding CAR had superior antigen-specific suppression, proliferation and cytokine production. In contrast, in vivo protection from skin allograft rejection and DSAs production was similar between Tregs expressing CARs encoding CD28, ICOS or PD1, but not GITR, 41BB or OX40, co-stimulatory domains. To reconcile in vitro and in vivo data, the in vivo effects of a CAR encoding CD3ζ but no co-stimulatory domain were studied, revealing protective effects similar to the CD28-encoding CAR.
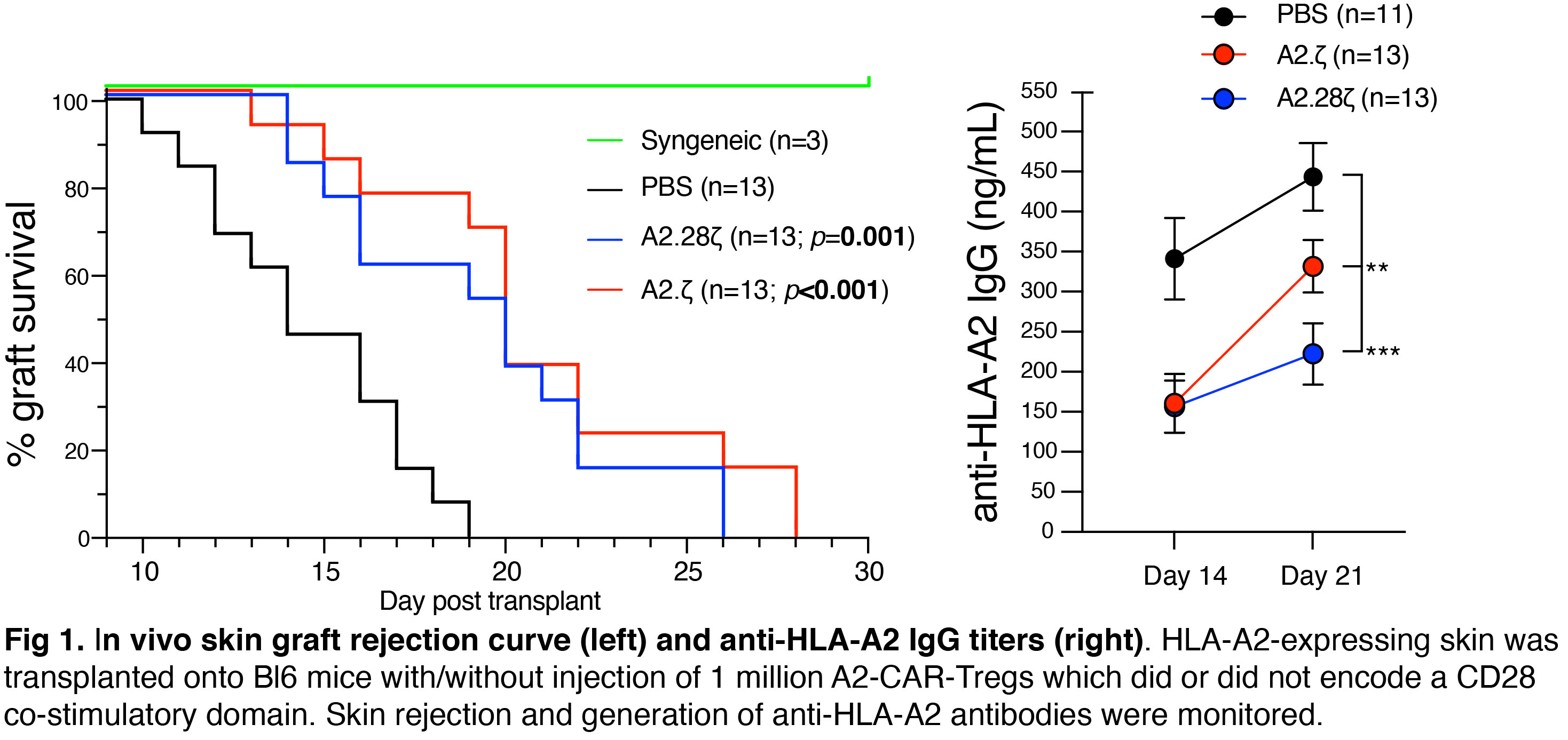
To interpret these results, we explored if CAR-independent co-stimulation modulates CAR-Treg function. We found that exogenous co-stimulation from APCs compensates for the lack of a CAR-encoded CD28 domain. To exclude the possibility that our observations could be related to dimerization between native CD28 and the CAR-encoded CD28-transmembrane domain (TM), we generated new CARs encoding a CD8a-derived TM. CAR-Tregs with CD8a-TM domains were similarly able to respond to exogenous stimulation suggesting a minimal impact of the type of TM in this process.
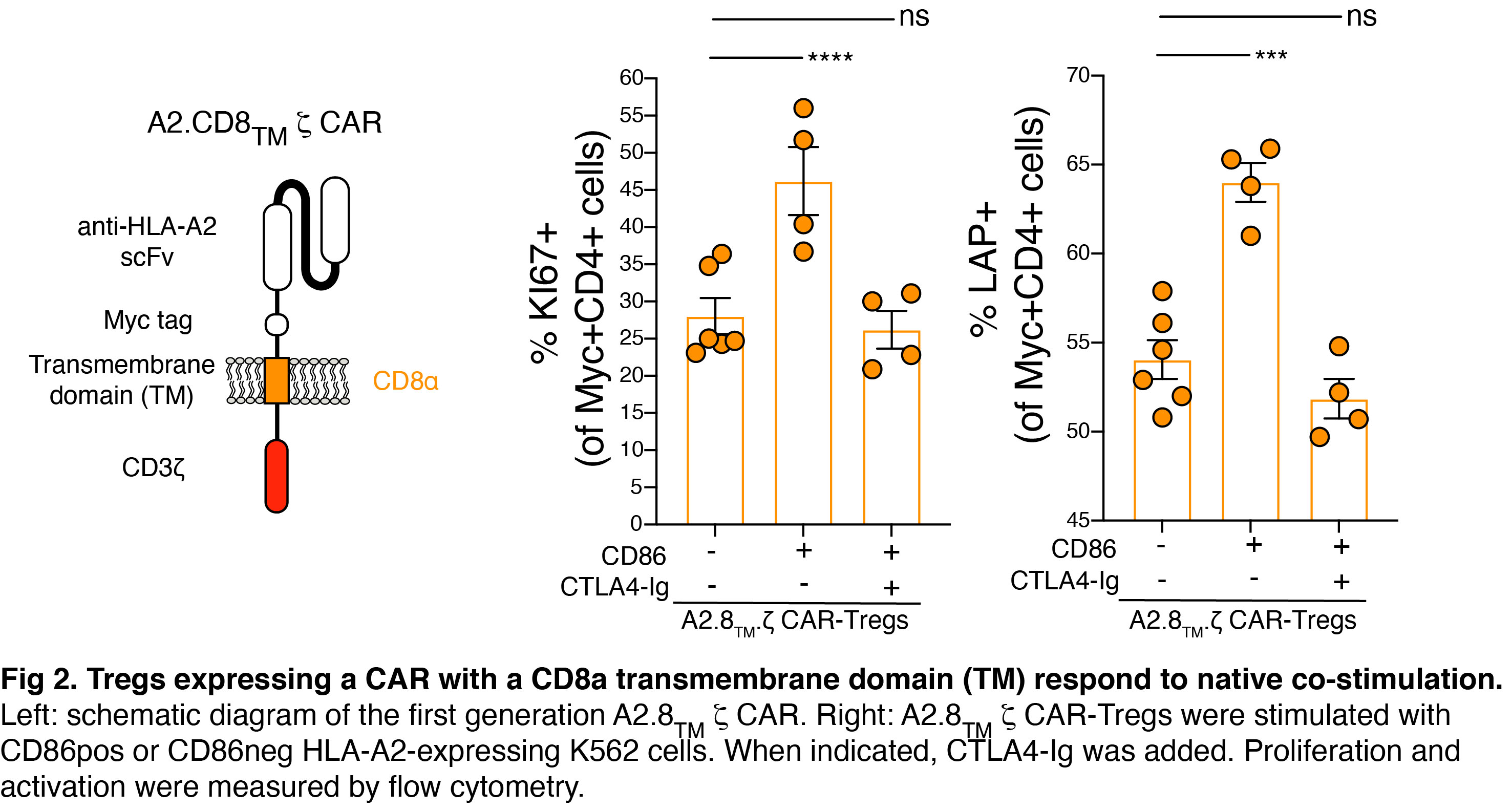
Conclusions: Tregs expressing a CAR with or without CD28 are functionally equivalent in vivo, likely due to co-stimulation provided by endogenous APCs. This study reveals a new dimension of CAR-Treg biology and has important implications for the design of CARs for clinical use in Tregs.
Supported by grants from the US Department of Defense (W81XWH-19-1-0351) and the Canadian Institutes of Health Research (FDN-154304). IRS and DAB are supported by salary awards from the Canadian Institutes for Health Research and Michael Smith Health Research BC. MKL receives a salary award from the BC Children's Hospital Research Institute and is a Canada Research Chair in Engineered Immune Tolerance. Some figures were created with BioRender (http:/biorender.com) .
Lectures by Isaac Rosado Sanchez
| When | Session | Talk Title | Room |
|---|---|---|---|
|
Tue-02 14:25 - 15:30 |
Bioengineering for transplantation | Regulatory T cells integrate native and CAR-mediated co-stimulatory signals for control of allograft rejection | Grand Georgian |
