Postdoctoral fellow
General Surgery
BC Children’s Hospital Research Institute
Modelling HLA-A2 CAR-Treg therapy with a novel transgenic mouse
Manjurul Haque1, Paul C Orban1, Nicole Hofs2, Pamela Hoodless2, Megan K Levings1.
1General Surgery , BC Children’s Hospital Research Institute, Vancouver, BC, Canada; 2BC Cancer Research Centre, Vancouver, BC, Canada
Introduction: Regulatory T cell (Treg) therapy offers an alternative to immunosuppression after transplantation. Tregs modified to express a donor-specific chimeric antigen receptor (A2-CAR) delay rejection of HLA-A2+ skin and heart grafts and hold promise as a cellular therapy to reduce allograft rejection. Although this strategy has moved to first-in-human testing, many questions related to mechanisms of action, interaction with standard immunosuppressive therapy, and longevity/stability of the cell product remain. These questions can be addressed using mouse models of transplantation but the inefficiency of genetically-modifying mouse Tregs ex vivo by retroviral transduction is a limitation. We, therefore, created a new mouse model where A2-CAR expression on defined cell lineages is inducible with CRE expression.
Methods: We generated a construct where A2-CAR expression is driven by a synthetic CAG promoter and controlled by a Lox-flanked transcriptional stop-cassette. The cassette also encoded an IRES-mKO2 reporter. The construct was placed between homology arms of the Rosa 26 locus and introduced into C57 Bl/6 embryonic stem cells using CRISPR/Cas9. Successfully targeted ES cells were injected into goGermline blastocysts, which were implanted in pseudo-pregnant CD-1 mice. Upon confirming the presence of the transgene, chimeras were bred with C57 Bl/6 females. To confirm inducible expression of the A2-CAR, CD4+ T cells were transduced ex vivo with a CRE-encoding retrovirus in F1 mice. A2-CAR positive F1 mice were crossed with C57 Bl/6 Foxp3-Gfp-CreERT2 mice to generate a system where A2-CAR expression can be induced explicitly in Foxp3+ Tregs by tamoxifen injection.
Results: Five consecutive tamoxifen injections resulted in A2-CAR expression in GFP+FoxP3+ Tregs. A2-CAR-Tregs were sorted as CD4+CD25+GFP+mKO2+ cells and expanded on HLA-A2 expressing artificial antigen presenting cells. After 9 days, the numbers of A2-CAR-Tregs increased 10-20-fold and maintained >90% viability, and were >95% FoxP3+Helios+A2-CAR+. A2-CAR Tregs demonstrated antigen-specific suppression of HLA-A2-specific T cells in vitro and homed to A2+ grafts in vivo. Additionally, A2-CAR-Tregs mediated bystander suppression of OTII CD4+ T cells in the presence of Ova peptide.
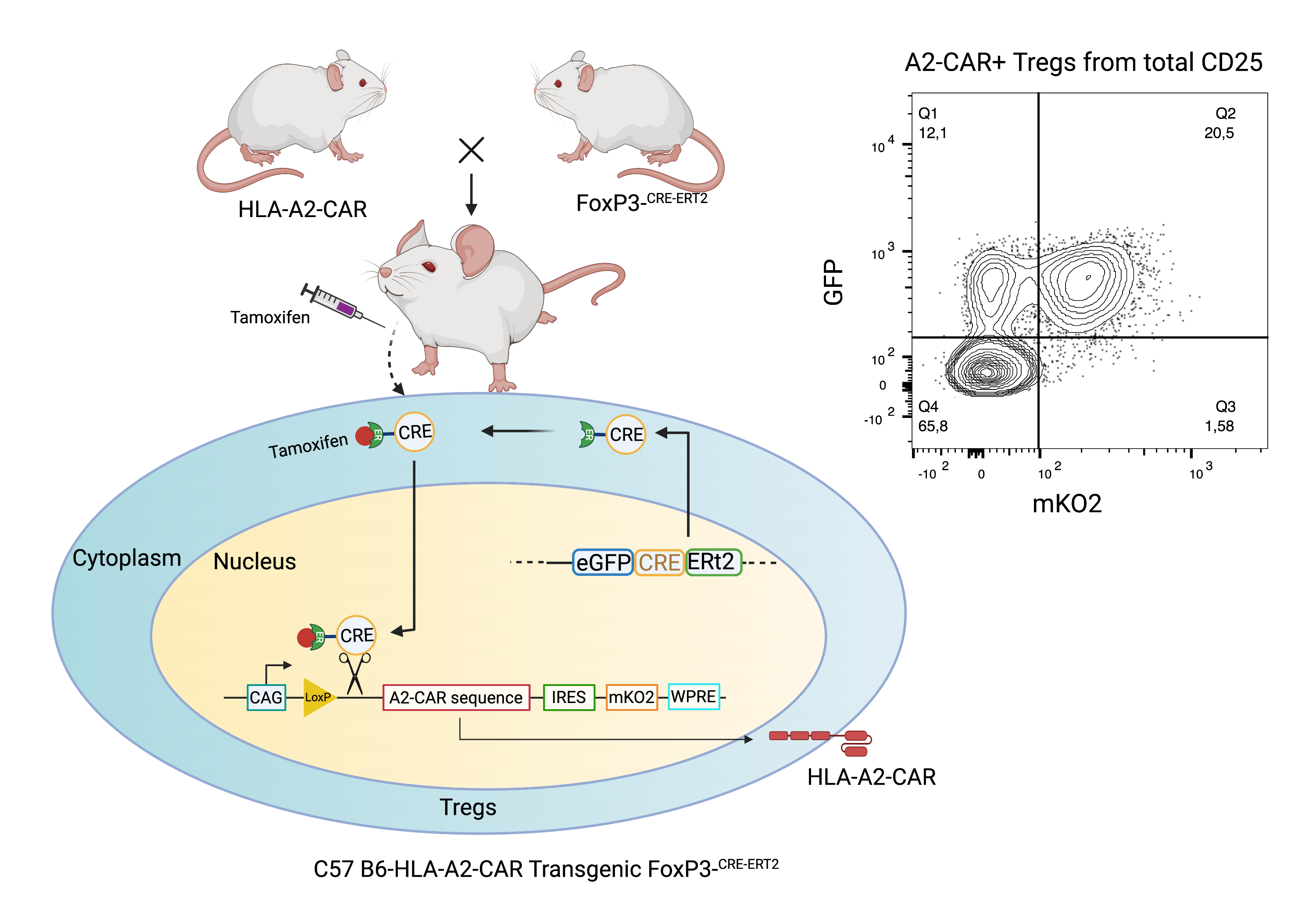
Conclusion: These results show that A2-CAR-Tregs from the new transgenic mouse line maintain the expected phenotype and function, and have the major advantaged of increased viability, expansion potential and homogeneous phenotype compared to cells made with retroviral transduction. These mice now enable in depth study of various aspects of A2-CAR Tregs biology to better understand and optimize their ability to control allograft rejection.
Lectures by Manjurul Haque
| When | Session | Talk Title | Room |
|---|---|---|---|
|
Tue-02 14:25 - 15:30 |
Bioengineering for transplantation | Modelling HLA-A2 CAR-Treg therapy with a novel transgenic mouse | Grand Georgian |
