Graduate Student
School of Biomedical Engineering
University of British Columbia
Development of an MHC Class II-specific CAR for the modulation of alloreactivity in mice
Andrew Brown1,2, Ismail Sayin3, Samarth S. Durgam3, Isaac Rosado-Sanchez1,2, Manjurul Haque1,4, Madeleine Speck1,4, Vivian Fung1,4, Majid Mojibian1,4, Anita S. Chong3, Megan K. Levings1,2,4.
1BC Children's Hospital Research Institute, Vancouver, BC, Canada; 2School of Biomedical Engineering, University of British Columbia, Vancouver, BC, Canada; 3Department of Surgery, University of Chicago, Chicago, IL, United States; 4Department of Surgery, University of British Columbia, Vancouver, BC, Canada
Introduction: Long-term outcomes in solid organ transplantation remain hindered by chronic rejection and toxicities associated with life-long immunosuppression. A potential solution is the induction of allograft tolerance through cell therapy with donor-specific regulatory T cells (Tregs). We and others have shown that Treg specificity can be re-directed by the expression of a chimeric antigen receptor (CAR) specific for MHC Class I1, and that CAR-Tregs specific for HLA-A2 are more potent than are polyclonal Tregs at ameliorating rejection of HLA-A2+ skin2-4 or heart5 grafts. However, a possible limitation of Class I-specific Tregs is the constitutive and ubiquitous expression of MHC Class I and thus possible Treg exhaustion due to chronic stimulation. Moreover, since many Treg suppressive mechanisms are mediate via antigen presenting cells it is possible that redirection of Treg specificity towards MHC Class II could be advantageous and provide opportunities for combination CAR-Treg therapy. To address this question, we generated a new CAR specific for MHC Class II.
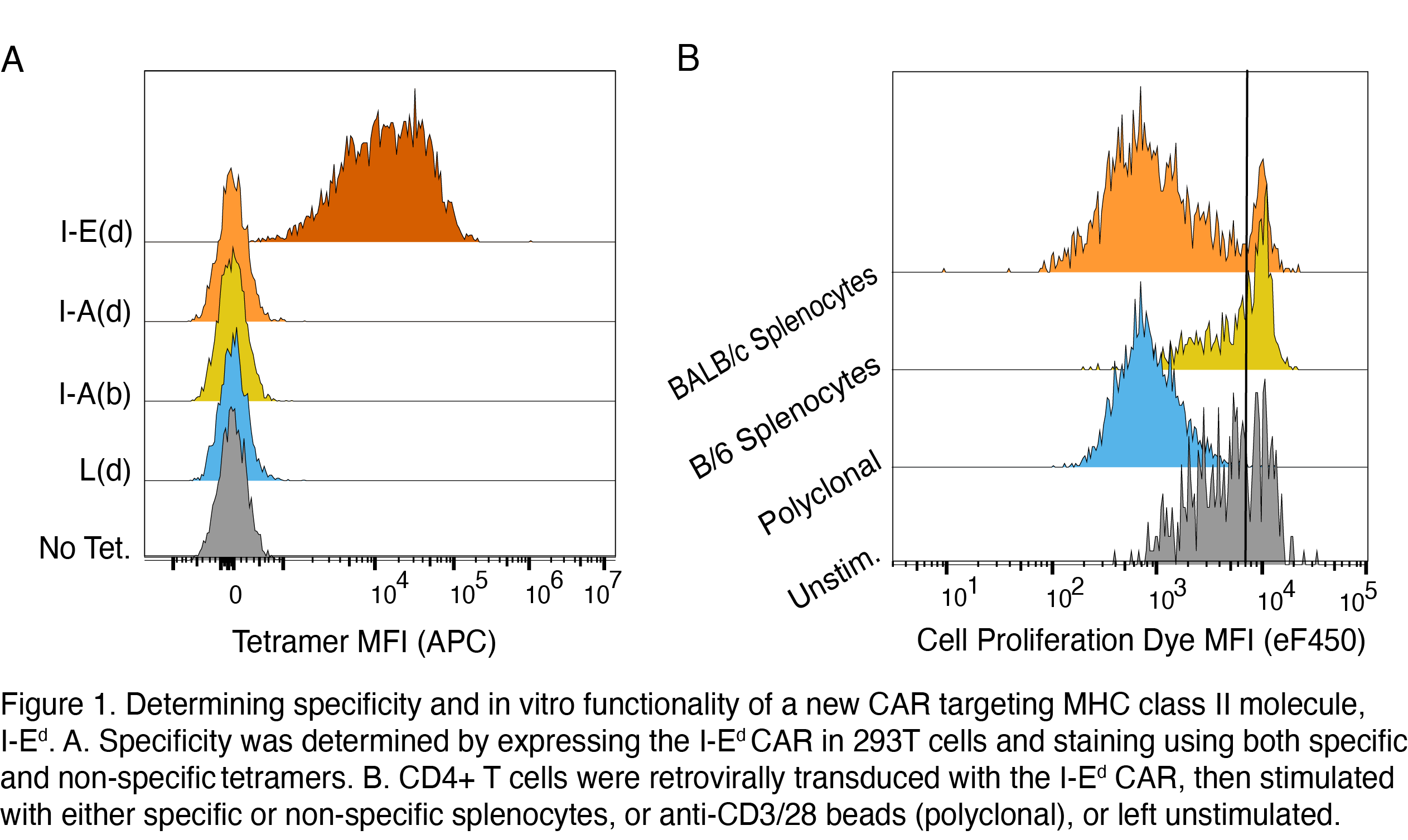
Methods: The heavy and light chains from a mAb specific for the I-Ed MHC class II molecule expressed by BALB/C mice were used to generated a single chain variable fragment which was then fused to intracellular sequences encoding CD28 and CD3zeta. CAR surface expression and ability to bind to specific/non-specific tetramers were tested by transient transfection in 293T cells. Function in T cells was determined by retroviral transduction of the I-Ed-CAR into CD4+ T cells from BL/6 mice, followed by stimulation with specific/non-specific splenocytes. A preliminary in vivo study involved transplantation of a BALB/C skin onto BL/6 mice and injection with/without I-Ed CAR-Tregs on the day of transplantation.
Results: The I-Ed CAR was successfully expressed on the surface of 293T cells and bound I-Ed tetramers, but not other MHC class I or II tetramers, indicating preservation of specificity (Fig. 1A). I-Ed CAR T cells from BL/6 mice were activated and proliferated in response to BALB/C splenocytes, but not to those from BL/6 mice (Fig. 1B). I-Ed CAR-Tregs were generated and 1 million were injected into BL/6 mice recipients of BALB/C skin. Longitudinal monitoring showed that the Tregs engrafted and remained Foxp3+ and CAR+. Experiments are ongoing to determine effects on skin rejection.
Conclusion: We created a new I-Ed-specific CAR and confirmed expression, antigen specificity and function. This new tool enables investigation of the function of MHCII-targeted CAR-Tregs in an immunocompetent model of allogeneic skin transplantation. These studies will contribute to the rational design of CAR-Treg therapies to improve outcomes in solid organ transplantation.
References:
[1] MacDonald, K.G., Hoeppli, R.E., Huang, Q., Gillies, J., Luciani, D.S., Orban, P.C., Broady, R. & Levings, M.K. Alloantigen-specific regulatory T cells generated with a chimeric antigen receptor. J Clin Invest 126, 1413-1424 (2016).
[2] Noyan, F., Zimmermann, K., Hardtke-Wolenski, M., Knoefel, A., Schulde, E., Geffers, R., Hust, M., Huehn, J., Galla, M., Morgan, M., Jokuszies, A., Manns, M.P. & Jaeckel, E. Prevention of Allograft Rejection by Use of Regulatory T Cells With an MHC-Specific Chimeric Antigen Receptor. Am J Transplant 17, 917-930 (2017).
[3] Boardman, D.A., Philippeos, C., Fruhwirth, G.O., Ibrahim, M.A., Hannen, R.F., Cooper, D., Marelli-Berg, F.M., Watt, F.M., Lechler, R.I., Maher, J., Smyth, L.A. & Lombardi, G. Expression of a Chimeric Antigen Receptor Specific for Donor HLA Class I Enhances the Potency of Human Regulatory T Cells in Preventing Human Skin Transplant Rejection. Am J Transplant 17, 931-943 (2017).
[4] Sicard, A., Lamarche, C., Speck, M., Wong, M., Rosado-Sanchez, I., Blois, M., Glaichenhaus, N., Mojibian, M. & Levings, M.K. Donor-specific chimeric antigen receptor Tregs limit rejection in naive but not sensitized allograft recipients. Am J Transplant 20, 1562-1573 (2020).
[5] Wagner, J.C., Ronin, E., Ho, P., Peng, Y. & Tang, Q. Anti-HLA-A2-CAR Tregs prolong vascularized mouse heterotopic heart allograft survival. Am J Transplant (2022).
Lectures by Andrew W. Brown
| When | Session | Talk Title | Room |
|---|---|---|---|
|
Tue-02 16:15 - 17:20 |
Abstracts Session 5 | Development of an MHC Class II-specific CAR for the modulation of alloreactivity in mice | Grand Georgian |
