Adriana Montalvan, United States
Post Doctoral Research Fellow
Transplant Surgery
Beth Israel Deaconess Medical Center
Outcomes of solid organ transplantation recipients after Tixagevimab/Cilgavimab treatment
Adriana Montalvan1, Ava Sanayei2, Julia Ochalla3, Isabella Faria1, Martha Pavlakis4, Michael Curry3, Behnam Saberi3.
1Transplant Surgery, Beth Israel Deaconess Medical Center, Boston, MA, United States; 2Internal Medicine, Beth Israel Deaconess Medical Center, Boston, MA, United States; 3Transplant Hepatology, Beth Israel Deaconess Medical Center, Boston, MA, United States; 4Transplant Nephrology, Beth Israel Deaconess Medical Center, Boston, MA, United States
Introduction: Even though vaccination against COVID-19 significantly reduced mortality and hospitalizations in the general population, solid organ transplant (SOT) recipients are particularly vulnerable to infection. Tixagevimab co-packed with Cilgavimab (Tix-Cil) was developed for COVID-19 pre-exposure prophylaxis for immunocompromised patients. However, data on the efficacy of this drug on SOT recipients is limited. We investigated the rates of COVID-19 positivity in liver, kidney, and pancreas recipients who received Tix-Cil.
Methods: We conducted a prospective single-center registry of liver, kidney, and pancreas transplant recipients who were treated with Tix-Cil in January 2022. Recipients of SOTs from our institution who declined Tix-Cil administration or had not received it by the study period served as the control group (non-Tix-Cil). Demographics, type of transplant, immunosuppression-related information, COVID-19 vaccination status, and Tix-Cil administration date were collected through telephone interviews and electronic medical records review. Patients were interviewed every month for up to six months or until the participants tested positive for COVID, whichever occurred earlier. Kaplan-Meier COVID-free survival curve was performed based on date of Tix-Cil administration and date of COVID-19 infection. Log-rank test was used for comparison. Univariate and multivariate Cox regression models were constructed.
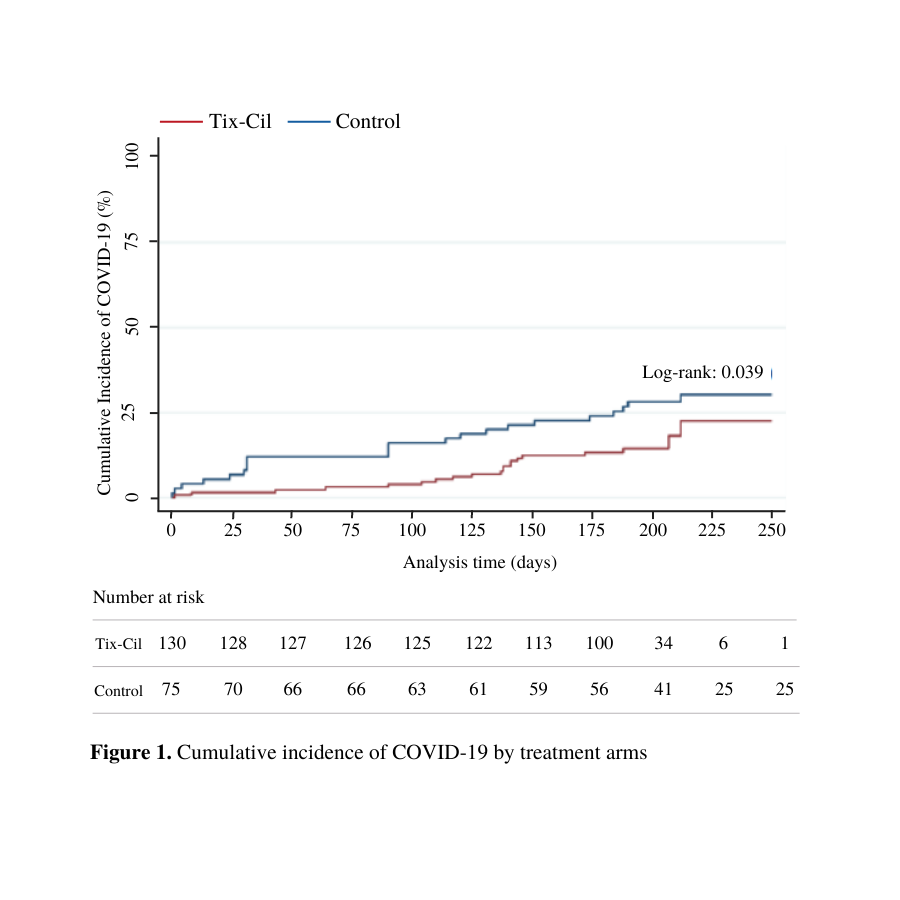
Results: The study cohort included 205 patients, of which 63.4% (n=130) received Tix/Cil, and 36.6% (n=75) served as controls. Overall, 62.0% were male, 73.7% White, with mean age of 59.6 (±12.5). Regarding the type of organ transplantation, there were 62.9% (n=129) liver, 20.5% (n=42) kidney, 11.2% (n=23) liver-kidney , and 5.4% (n=11) pancreas recipients. About half of the patients were on only one immunosuppression drug (Tix-Cil cohort: 48.3% and non-Tix-Cil cohort: 51.7%). The mean time post-transplantation was 5.9 (SD ±5.5) years in the Tix-Cil group vs. 8.1 (SD ±6.8) years in the control group (p=0.01). COVID-19 vaccination rate was significantly different among groups, with 99.2% (n=129) in the Tix-Cil vs. 92.0% (n=69) non-Tix-Cil cohort (p=0.006). There was no statistically significant difference in the rate of symptom presence or treatment administration in the infected patients from both cohorts (p>0.05). In multivariate analysis receiving Tix-Cil and time following transplant, in years, were associated with reduction in the risk of SARS-CoV-2 infection, hazard ratio of 0.482 (95% CI 0.246 - 0.943) and hazard ratio of 0.917 (95% CI 0.848 - 0.990), respectively.
Conclusion: During the study period, patients who received Tix-Cil had a significant reduction in the rate of SARS-CoV-2 infection. Even though no difference in clinical parameters such as symptomatology and treatment were found between the control and active group, the clinical implications of Tix-Cil remain a subject of study.
Lectures by Adriana Montalvan
| When | Session | Talk Title | Room |
|---|---|---|---|
|
Wed-03 09:20 - 10:10 |
Abstracts Session 6 | Outcomes of solid organ transplantation recipients after Tixagevimab/Cilgavimab treatment | Grand Georgian |
