Donor-derived cell-free DNA and gene expression profile in simultaneous pancreas-kidney transplantation
Maria Jose Ramirez-Bajo1,2, Jordi Rovira1,2, Elisenda Banon-Maneus1,2, Natalia Hierro-Garcia1,2, Marta Lazo-Rodriguez1,2, Miriam Cuatrecases3, Maria Angeles Garcia-Criado4, Maria Paula Gomez5, Carolina Oliva5, Ettore Cotroneo6, Riccardo Giannico6, Graziella Calugi6, Juston Weems7, Federic Cofan1,2,8, David Cucchiari1,8, Nuria Esforzado1,8, Enrique Montagud-Marrahi1,8, Federic Oppenheimer1,2,8, Gaston Piñeiro1,8, Ignacio Revuelta1,2,8, Vicens Torregrossa1,2,8, Jessica Ugalde1,8, Antonio J Amor9, Joana Ferrer, Josep M Campistol, Fritz Diekmann, Pedro Ventura-Aguiar.
1Laboratori Experimental de Nefrologia i Trasplantament, Fundacio de Recerca Clínic Barcelona –Institut d’Investigacions Biomediques August i Sunyer, Barcelona, Spain; 2Red de Investigación Renal (REDINREN), Madrid, Spain; 3Pathology, Biomedical Diagnosis Center, Hospital Clinic, Barcelona, Spain; 4Radiology, Imaging Diagnosis Center, Hospital Clinic, Barcelona, Spain; 5Eurofins Megalab Clinical Genetics Laboratory, Madrid, Spain; 6Eurofins Genoma Group, Molecular Genetics Laboratories, Rome, Italy; 7Eurofins Transplant Genomics, Framingham, MA, United States; 8Nephrology and Kidney Transplant, Renal Transplant Unit, Hospital Clinic, Barcelona, Spain; 9Endocrinology and Nutrition, Hospital Clinic, Barcelona, Spain; 10Hepato-bilio-pancreatic and Surgery and Digestive Transplant , Hospital Clinic, Barcelona, Spain
Background: Rejection is the major cause of graft failure after pancreas transplantation, but pancreas graft biopsies are a challenging and risky technique. Molecular biomarkers, such as donor-derived cell-free DNA (dd-cfDNA) and gene expression profile (GEP), have emerged as an alternative to monitor graft rejection in other solid organ transplants. We hypothesized whether these techniques could complement the noninvasive diagnostic performance of currently available biomarkers of pancreas graft dysfunction.
Methods: We performed a longitudinal analysis of stored plasma samples from 38 pancreas transplant recipients during the first years. Plasma samples were collected in PAXgene tubes pre-transplant, and afterwards at 1h, 24h and 7 days post-transplant, and at time of pancreas biopsy – either per protocol at 3 weeks and 12 months, or for clinical indication. The Allonext assay (Eurofins Genoma Group) was used to report the dd-cfDNA as a percentage of the donor-derived fraction as compared with total cfDNA by using panels of more than 500 SNPs . GEP was analyzed with the TruGrafâ algorithm—a gene expression algorithm analyzing differential expression of 120 genes. GEP results were provided as a probability score normalized on a 0–100 scale. The TruGrafâ assay (EurofinsTransplant Genomics) has a previously defined probability threshold of 50 to differentiate the TX (no rejection) from the not TX phenotype in kidney transplantation.
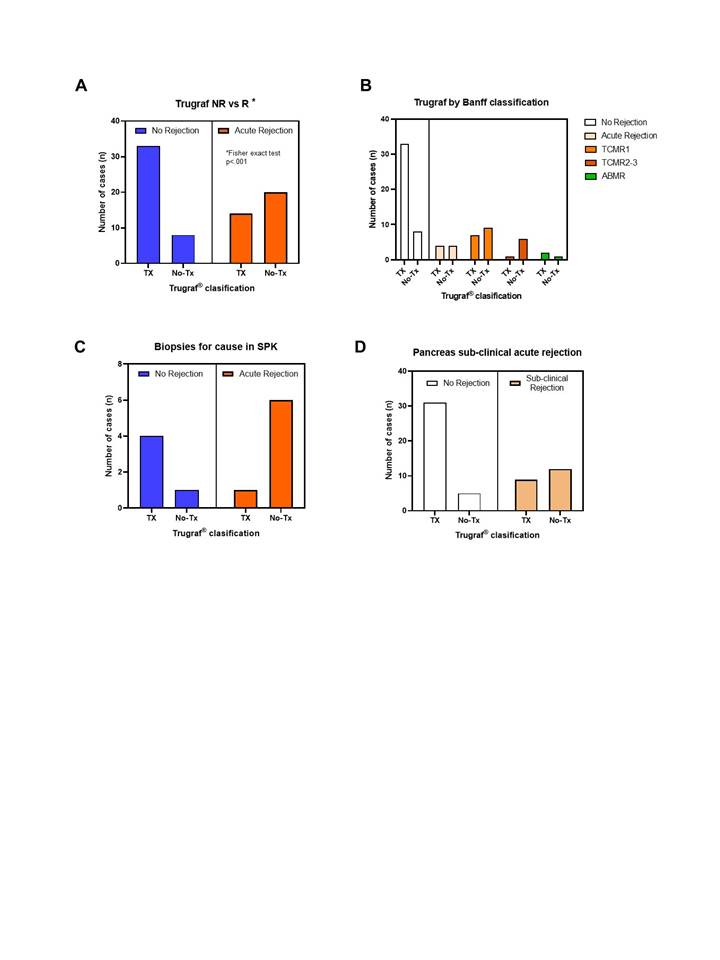
Results: Dd-cfDNA increased significantly during the first hour after pancreas transplantation (4.30±2.53%), and reduced progressively (at 24h 2.41±1.79%, at 1 week 0.65±0.47%, at 3 weeks 0.47±0.28%). Patients with biopsy-proven acute rejection (P-BPAR) episodes presented higher values of dd-cfDNA (2.1±2.46% vs 0.50±0.88%; p<0.027), most prominently in those with acute rejection diagnosed beyond 30 days from transplant.
In patients with pancreas acute rejection, GEP diagnosed the rejection episode in 58.8%, presenting a sensitivity of 74.1% (95% CI 53.7-88.9) and a specificity of 83.3% (95% CI 59.8-92.5) for the diagnosis of acute rejection, with negative predictive value of 86.7% (95% CI 77.3-92.6). Sub-clinical rejection (lipase <3xs normal) was diagnosed by GEP in 57.1%, with a specificity of 86.1% (95% CI 71-95). The combination of both parameters presented a negative predictive value of 82.3% (67-91%), with thirteen patients with P-BPAR (72.2%) presenting either test positive.
Conclusions: Dd-cfDNA and GEP can improve the detection of pancreas acute rejection episodes. GEP may provide non-invasive monitoring for sub-clinical rejection of the pancreas graft.
Lectures by Pedro Ventura-Aguiar
| When | Session | Talk Title | Room |
|---|---|---|---|
|
Wed-03 09:20 - 10:10 |
Abstracts Session 6 | Donor-derived cell-free DNA and gene expression profile in simultaneous pancreas-kidney transplantation | Grand Georgian |
