Arianna Barbetta, United States
Graduate research assistant
Surgery, Abdominal organ transplantation
University of Southern California
Interplay between intrahepatic macrophages and alloreactive and regulatory T-cells mediates the complex alloimmune microenvironment during acute rejection in clinical liver transplantation
Arianna Barbetta1, Brittany Rocque1, Sarah Bangerth1, Carly Weaver2, Janet Kim1, Bryce Roper1, Shefali Chopra3, Rohit Kohli4, Juliet Emamaullee1.
1Surgery, Division of Abdominal Organ Transplantation, University of Southern California, Los Angeles, CA, United States; 2Division of Hepatobiliary and Abdominal Organ Transplantation Surgery, Children's Hospital-Los Angeles, Los Angeles, CA, United States; 3Pathology, University of Southern California, Los Angeles, CA, United States; 4Pediatrics, Children's Hospital-Los Angeles, Los Angeles, CA, United States
Background: Acute T-cell mediated rejection (TCMR) remains the most frequent complication after liver transplantation (LT). Features of innate and adaptive components of alloimmunity within the human allograft are poorly understood. Using Imaging Mass Cytometry (IMC), a multiplexed imaging-based single-cell technology, we sought to characterize the intrahepatic cellular profiles and interactions in TCMR in clinical LT.
Methods: Liver specimens from 76 patients including 38 with TCMR (RAI≽ 4), 14 with chronic rejection (CR), and 24 with no-rejection (NR) were selected retrospectively from our center. Multiplexed images of FFPE slides were obtained using a 22 IMC antibody panel (17 immune and 5 structural markers). Supervised and unsupervised clustering were used to aggregate single-cell data based on marker expression and for cluster identification. Cell-to-cell spatial interactions were analyzed via neighborhood analysis.
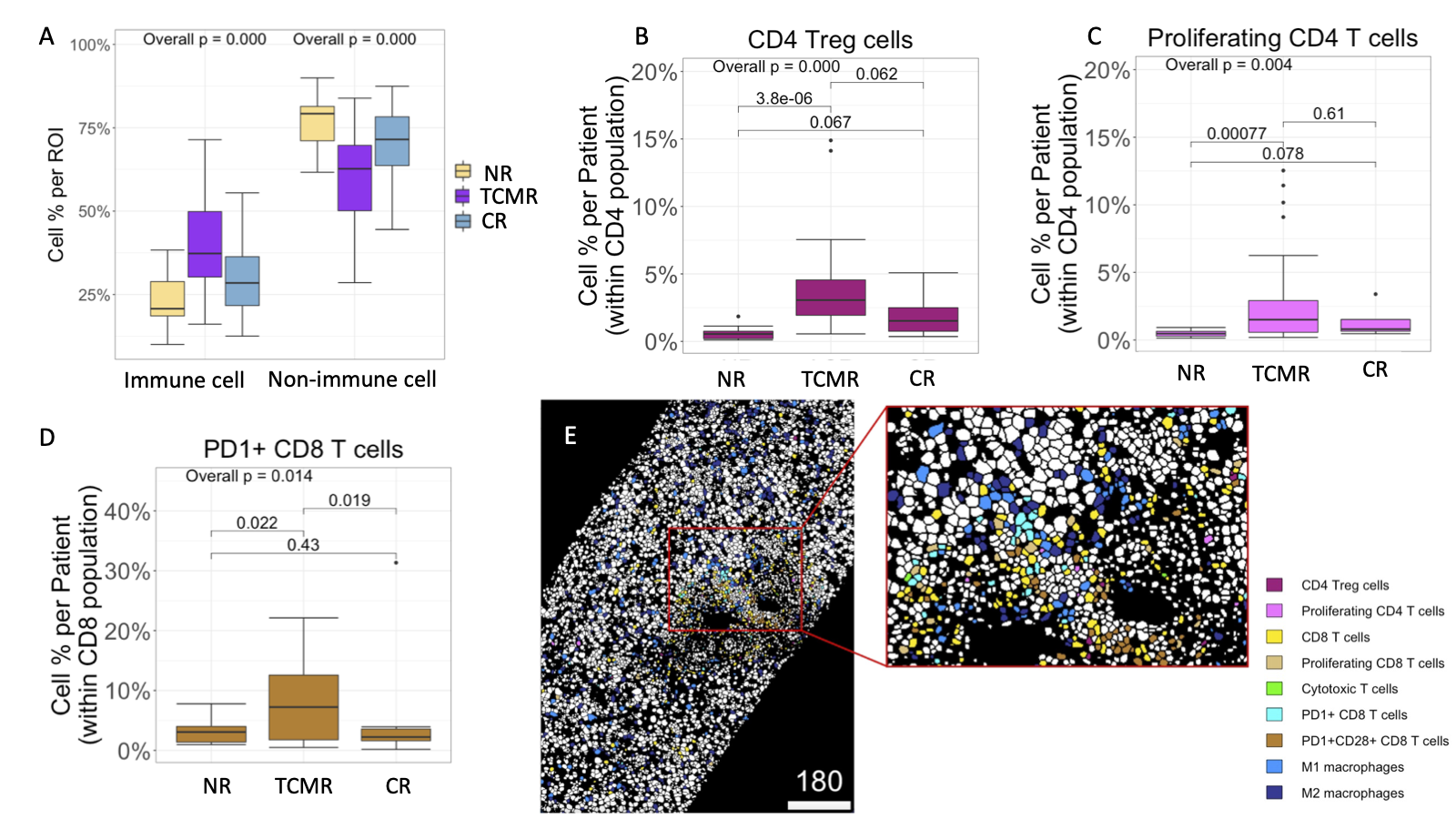
Results: Out of 461,816 cells identified across the entire cohort, 149,272 were classified as immune cells composed of 29 discrete subpopulations. TCMR had a greater proportion of immune cells compared to NR and CR (p<0.001) (Fig. 1A). Subcluster analysis within the CD3+CD4+ T-cell population showed an expansion of CD4+FoxP3+ Treg and proliferating CD4+ T-cells (Fig. 1B/C), with a higher ratio of Tregs:Proliferating CD4+ T-cells in TCMR compared to NR and CR (p=0.05). TCMR showed a greater proportion of PD1+CD8+ T-cells (Fig. 1D) and a higher ratio of PD1+CD8+T cell:Cytotoxic T-cells compared to CR and NR groups (p= 0.01), with more proliferating M1 and M2 macrophages (p<0.01 and p=0.034, respectively). Interestingly, in the context of TCMR, neighborhood analysis showed avoidance between macrophages and all CD8+Tcell subpopulations (p=0.01) (Fig 1E).
Conclusion: These data demonstrate that expansion of alloreactive T-cells in TCMR is associated with upregulation of regulatory phenotype CD4+Foxp3+ and PD1+CD8+ T-cells with a significant loss of interaction between M1/M2 macrophages and CD8+ T-cells. Therapies promoting expansion and interaction of intrahepatic macrophages and PD-1+ regulatory T-cells should be investigated as novel therapeutic targets for TCMR post-LT.
BR has received research support from a Broad Clinical Research Fellowship and One Legacy Fellowship Training Grant. JE was supported by a K08 from National Cancer Institute (K08CA245220), American Society of Transplant Surgeons Faculty Development Grant, Society for the Study of Liver Diseases, Clinical, Translational, and Outcomes Research Award, and Liver Scholar Award from Gilead Research Foundation.
Lectures by Arianna Barbetta
| When | Session | Talk Title | Room |
|---|---|---|---|
|
Wed-03 11:00 - 12:35 |
New perspectives in innate/ILC immunity and tissue-resident leukocytes | Interplay between intrahepatic macrophages and alloreactive and regulatory T-cells mediates the complex alloimmune microenvironment during acute rejection in clinical liver transplantation | Grand Georgian |
