Interclonal help promotes allospecific CD8+ T cell avidity maturation during graft rejection
Peter Wang1, Luqiu Chen1, Christine M McIntosh1, Husain Sattar2, Anita S Chong3, Maria-Luisa Alegre1.
1Medicine-Rheumatology, University of Chicago, Chicago, IL, United States; 2Pathology, University of Chicago, Chicago, IL, United States; 3Surgery-Transplant, University of Chicago, Chicago, IL, United States
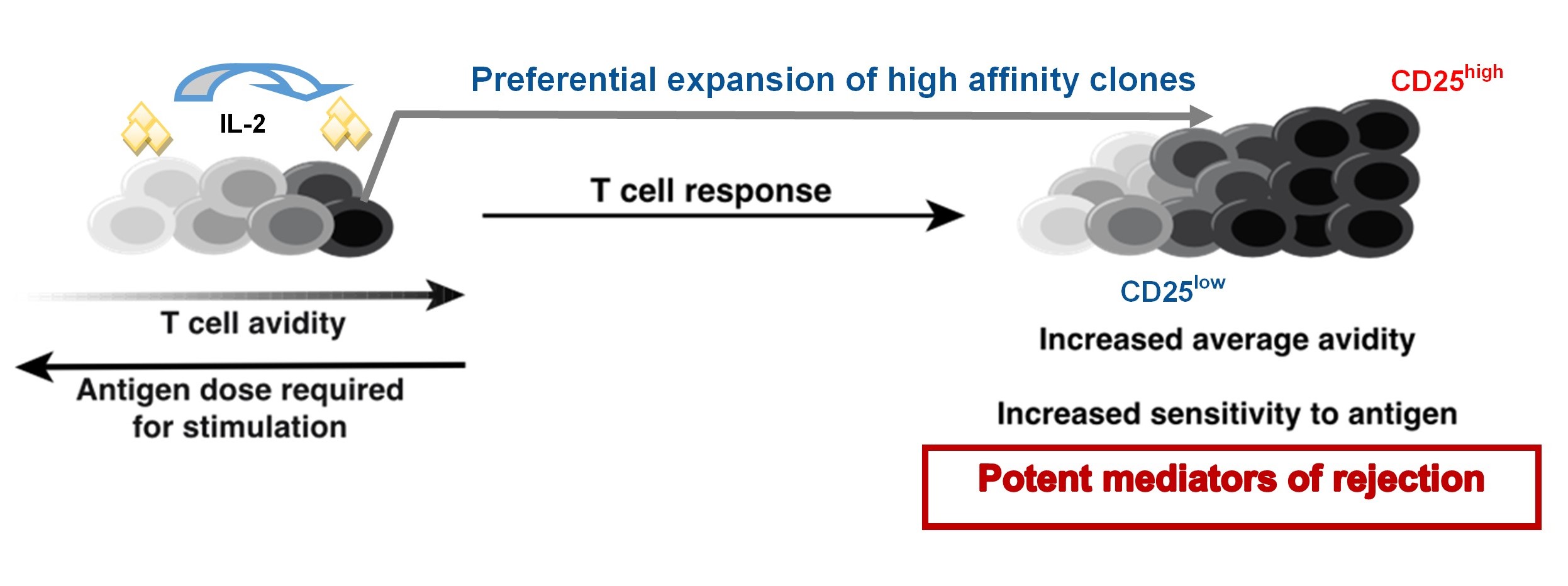
Introduction: During graft rejection, endogenous allospecific CD8+ T cell populations preferentially expand their high affinity clones, a process termed ‘avidity maturation.’ Expansion of these clones from a small precursor population results in a T cell population highly sensitive to alloantigen and poses a significant risk to the graft. From studies in anti-tumor and anti-viral/bacterial immunity, the central mechanism of T cell avidity maturation has predominantly been attributed to competition for limited antigen presented by antigen-presenting cells (APCs). However, because we observed the acquisition of an antigen-experienced phenotype by allospecific CD8 T cell clones of lower affinity following transplantation, we investigated whether other mechanisms might explain CD8+ T cell avidity maturation.
Methods: To investigate the fate, phenotype, and function of T cell populations with different affinities for alloantigen, we used congenically-marked CD8+ TCR-transgenic T cells with high (OT-1) and low affinity (OT-3) for a model alloantigen ovalbumin (OVA) which were adoptively transferred into mice transplanted with OVA-expressing skin grafts. In parallel, we tracked the endogenous polyclonal CD8+ T cell response to OVA using fluorescently labeled pMHC tetramer staining, monitored the kinetics of skin graft rejection, and performed histological analysis to quantify tissue damage.
Results: Low affinity CD8+ T cell clones were only modestly outcompeted by their high affinity clones for pMHC access, and rather, provided help for the expansion of their high affinity counterparts. Shortly after activation in vitro and in vivo, high affinity CD8+ T cell populations expressed increased levels of CD25 and better competed for the growth/proliferative factor IL-2 produced by all T cell clones shortly after activation. Transwell studies supported the notion that CD8-CD8 T cell help was delivered in a contact-independent manner and induced further CD25 upregulation. Finally, this “helped” expansion that drove the preferential, and more robust, accumulation of high affinity clones shortly after alloantigen encounter fueled increased alloimmunity and more severe graft damage.
Conclusion: Our results challenge the conventional paradigm that CD8+ T cell avidity maturation occurs solely through interclonal competition for cognate antigen based on TCR affinity. Rather, we show that ‘interclonal CD8-CD8 help,’ where lower/intermediate affinity clones help the preferential expansion of high affinity clones in an IL-2-dependent manner, results in a population that is more potent at driving rejection. Future studies will focus on the spatial/temporal cues that drive interclonal CD8-CD8 help and its implications on per-cell functionality or memory development.
This study was supported by NIH grants to MLA and ASC (NIAID P01AI-97113). We would like to acknowledge the staff of the UChicago Flow Cytometry Core (RRID: SCR_017760) and Animal Resources Facility (RRID:SCR_021806C) for their technical assistance.
Lectures by Peter Wang
| When | Session | Talk Title | Room |
|---|---|---|---|
|
Sun-30 16:40 - 18:00 |
Abstracts Session 1 | Interclonal help promotes allospecific CD8+ T cell avidity maturation during graft rejection | Grand Georgian |
