Masters of Science student
Department of Medicine
University of British Columbia
Development of a potency assay for regulatory T cell therapy applications
Macyn Leung1,2, Sabine Ivison2,3, Katherine N MacDonald2,4, Lieke Sanderink2,3, Megan K Levings2,3,4.
1Department of Medicine, University of British Columbia, Vancouver, BC, Canada; 2Childhood Diseases, BC Children’s Hospital Research Institute, Vancouver, BC, Canada; 3Department of Surgery, University of British Columbia, Vancouver, BC, Canada; 4School of Biomedical Engineering, University of British Columbia, Vancouver, BC, Canada
Introduction: Regulatory T cells (Tregs) are a promising cell therapy for the prevention of rejection and graft versus host disease after transplantation. Clinical trials have demonstrated Treg adoptive cell transfer to be safe, however the lack of a standardized functional assay to measure and compare the potency of different Treg products makes it difficult to compare study results and refine products for optimal in vivo efficacy. Moreover, potency assays are highly desirable for regulatory agencies. In vitro suppression of T cell proliferation is the current gold standard for measuring Treg function, but the current protocols are highly variable and the key points of technical versus biological variation are undefined. We thus aimed to standardize and optimize a Treg suppression assay.
Methods: Tregs were isolated and expanded from discarded human thymus. Responder targets of suppression were allogeneic peripheral blood mononuclear cells (PBMCs) or CD3-enriched PBMCs. We first tested how commonly varied steps and reagents – such as time of co-culture, activation reagents, media, and types of responder cells – affected assay reproducibility and feasibility. We then tested the assay’s capacity to distinguish between suppression mediated by activated Tregs versus conventional T cells, and incorporated transendocytosis of CD80 and CD86 from B cells as one of the assay readouts.
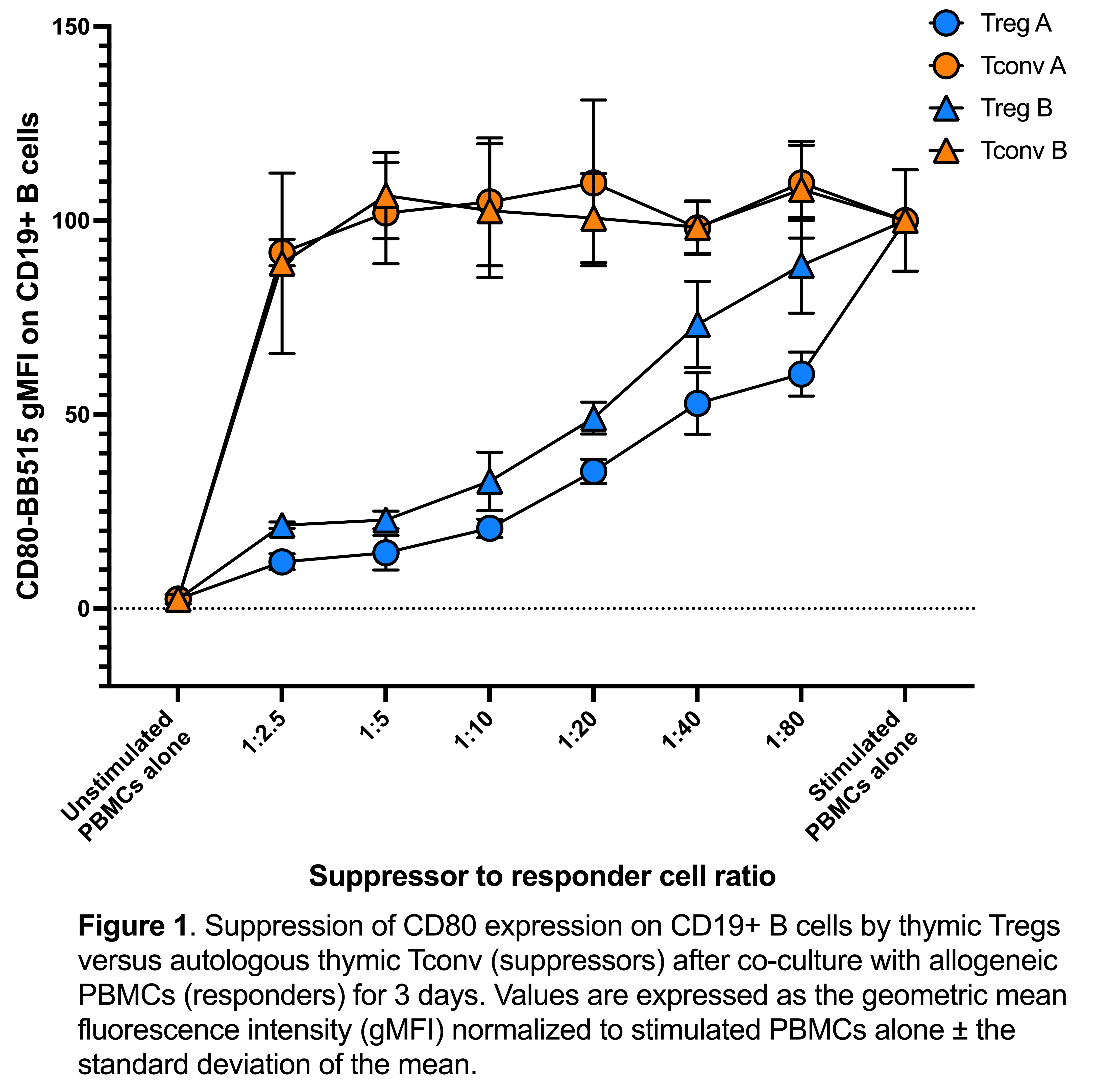
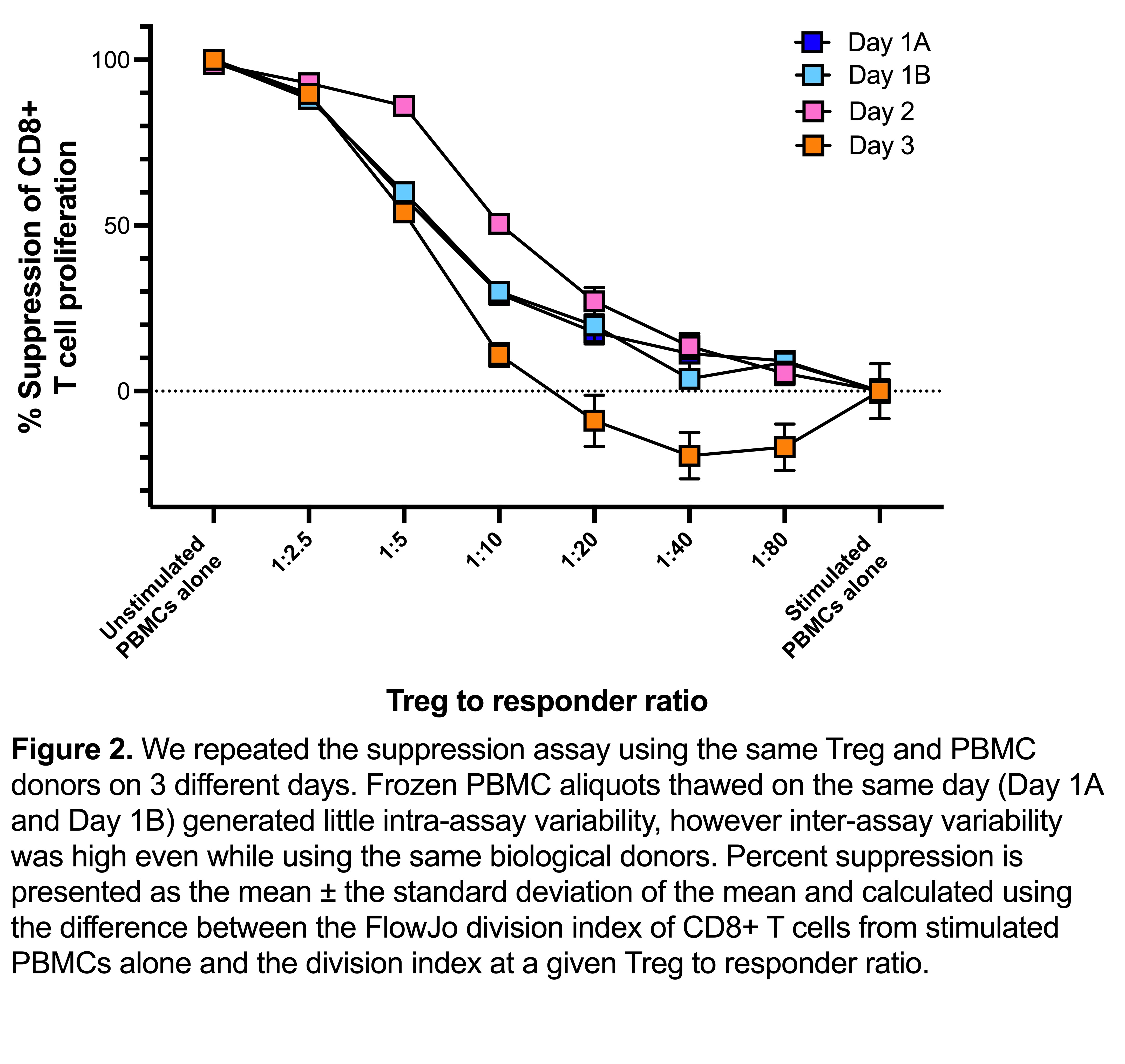
Results: We found the optimal conditions for reproducible responder T cell proliferation was activation for 3 days with Dynabeads Human T-Expander CD3/CD28. Media comparisons revealed that optimal Treg suppression required the addition of human serum to serum-free media; suppression assays in the absence of serum showed poor Treg suppression even at high Treg-to-PBMC ratios (e.g., 1:2.5). Measuring suppression of T cell proliferation was poorly able to distinguish between suppression mediated by activated Tregs versus inhibitory effects of activated conventional T cells. In contrast, transendocytosis of CD80 and CD86 on B cells (gated as CD19+ cells in PBMCs) was only mediated by Tregs and not by activated CD4+ conventional T cells. Interestingly, assay reproducibility was more strongly affected by intra-experimental factors (i.e. different experimental days) than a biological variation due to the use of Tregs or PBMCs from different individuals.
Conclusion: Measurement of CD80 and CD86 transendocytosis can be readily incorporated into Treg suppression assays and is a more specific measure of Treg function compared to suppression of T cell proliferation. Suppression assay variation can be reduced by standardizing media, activation reagents, and responder cell type, thus bringing us closer to fulfilling the critical need for a potency assay to test the efficacy of Treg therapies.
The authors are thankful for the volunteers, patients and their parents for contribution of samples along with the surgical and cardiac clinic staff at the British Columbia Children's Hospital who made this study possible, with special thanks to Allison Jamieson, Melanie Ganshorn, Aliyah Hassan, Lyn Nguyen and Colleen Ring.
References:
[1] McMurchy AN, Levings MK. Suppression assays with human T regulatory cells: a technical guide. European journal of immunology. 2012 Jan;42(1):27-34.
[2] MacDonald KN, Hall MG, Ivison S, Gandhi S, Geltink RI, Piret JM, Levings MK. Consequences of adjusting cell density and feed frequency on serum-free expansion of thymic regulatory T cells. Cytotherapy. 2022 Nov 1;24(11):1121-35.
[3] Sawitzki B, Harden PN, Reinke P, Moreau A, Hutchinson JA, Game DS, Tang Q, Guinan EC, Battaglia M, Burlingham WJ, Roberts IS. Regulatory cell therapy in kidney transplantation (The ONE Study): a harmonised design and analysis of seven non-randomised, single-arm, phase 1/2A trials. The Lancet. 2020 May 23;395(10237):1627-39.
[4] Wiesinger M, Stoica D, Roessner S, Lorenz C, Fischer A, Atreya R, Neufert CF, Atreya I, Scheffold A, Schuler-Thurner B, Neurath MF. Good manufacturing practice-compliant production and lot-release of ex vivo expanded regulatory T cells as basis for treatment of patients with autoimmune and inflammatory disorders. Frontiers in immunology. 2017 Oct 26;8:1371.
[5] Hou TZ, Qureshi OS, Sansom DM. Measuring CTLA-4-dependent suppressive function in regulatory T cells. Immunological Tolerance. 2019 (pp. 87-101).
Lectures by Macyn L Leung
| When | Session | Talk Title | Room |
|---|---|---|---|
|
Sun-30 16:40 - 18:00 |
Abstracts Session 1 | Development of a potency assay for regulatory T cell therapy applications | Grand Georgian |
