BC Children's Hospital Research Institute
CRISPR-mediated deletion of PD1 enhances the function of human CAR Tregs
Sonya Mangat1,2, Dominic A Boardman2,3, Jana K Gillies2,3, Andrew Brown2,4, Avery J Lam5, Megan K Levings2,3,4.
1Department of Microbiology and Immunology, University of British Columbia, Vancouver, BC, Canada; 2BC Children's Hospital Research Institute, Vancouver, BC, Canada; 3Department of Surgery, University of British Columbia, Vancouver, BC, Canada; 4School of Biomedical Engineering, University of British Columbia, Vancouver, BC, Canada; 5Department of Pathology, Stanford University, Stanford, CA, United States
Introduction: Graft rejection is currently managed using life-long, drug-based immunosuppression but these regimens have significant side-effects and limited success. Regulatory T cell (Treg) therapy offers more targeted immunosuppression with long-lasting benefits and is proven to be well-tolerated in clinical settings. However, questions remain regarding efficacy, in part due to a lack of antigen specificity. Allospecific Tregs can be generated through transduction with chimeric antigen receptors (CARs), synthetic fusion proteins that contain an antibody-derived antigen recognition domain to redirect Treg specificity towards donor-derived antigens. We and others have previously demonstrated that CARs dramatically improve the therapeutic potency of Tregs in mouse models of transplantation, yet indefinite graft survival has not been achieved. To further improve the efficacy of CAR Tregs and prolong graft survival, we aim to use CRISPR technology to delete PD1, a well-established inhibitory receptor that counteracts Treg activation.
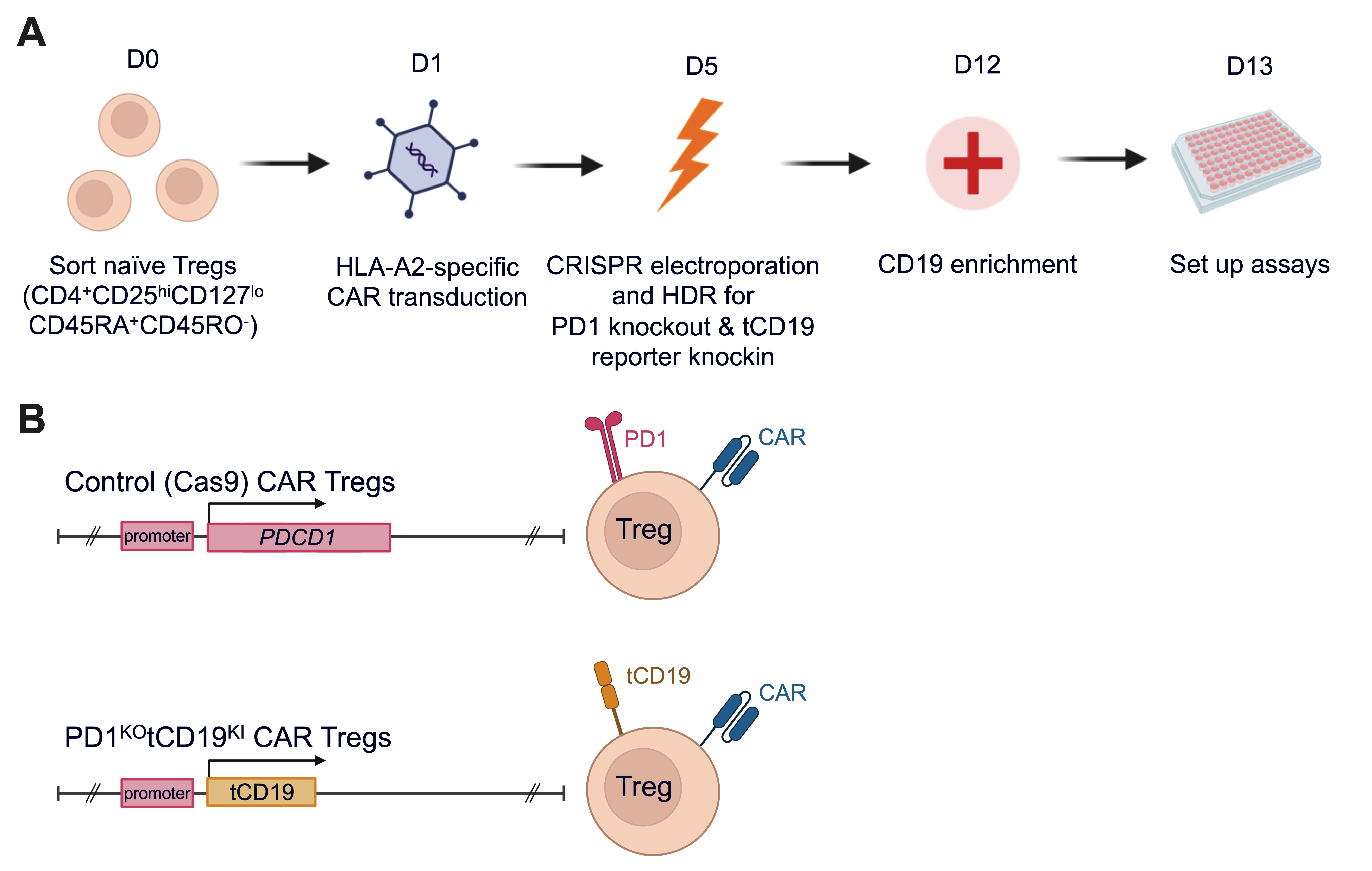
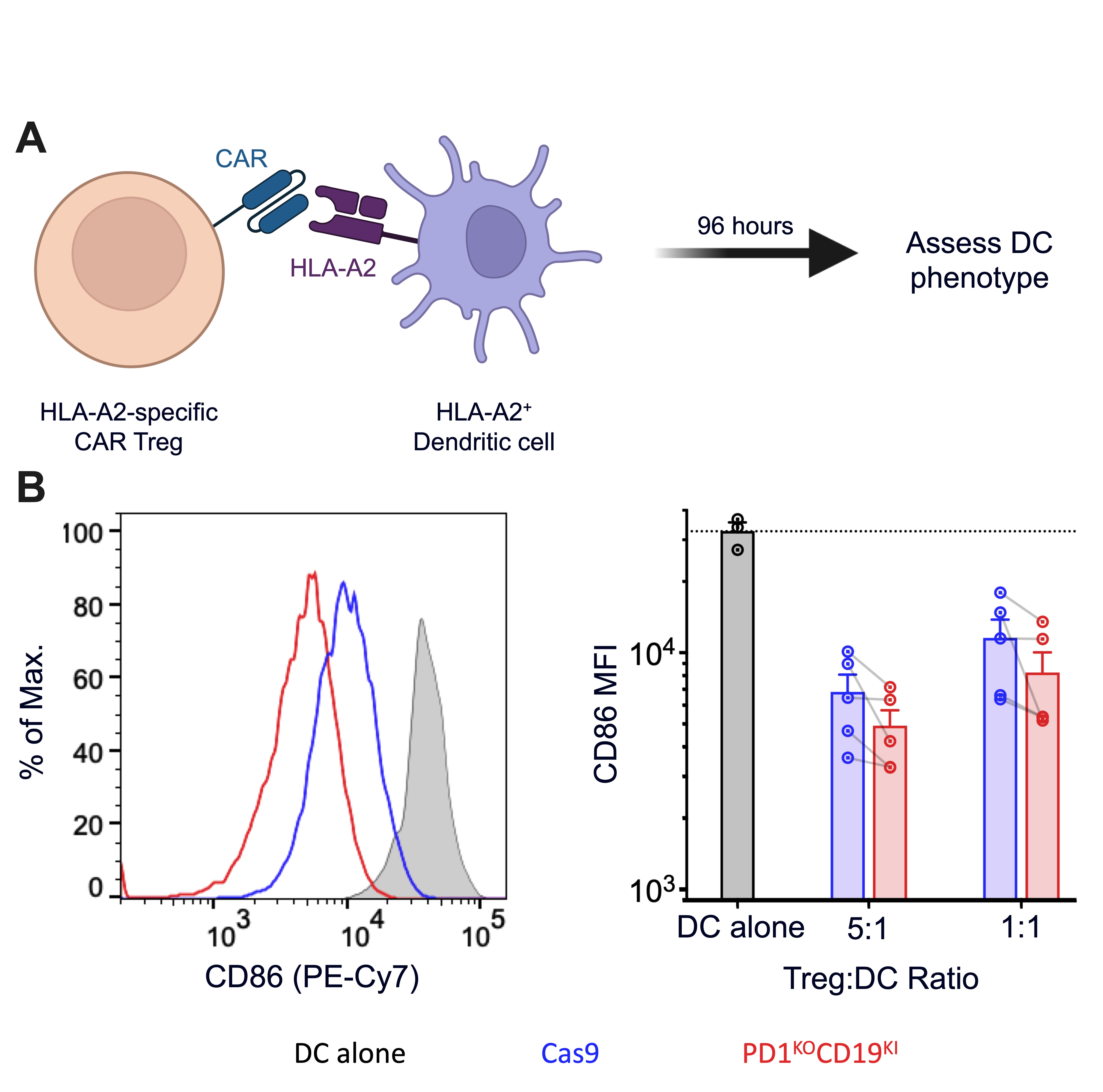
Methods: Naïve human Tregs were isolated from peripheral blood by cell sorting, and transduced with lentivirus to express an HLA-A2 specific CAR (Figure 1). PD1 was removed using CRISPR and successfully edited cells were identified by incorporation of a homology directed repair DNA template that encoded a truncated CD19 (tCD19) reporter to create PD1KOtCD19KI CAR Tregs. Control cells were electroporated with Cas9 protein alone. Cell phenotypes were assessed by flow cytometry and the functional consequences of PD1 removal were determined by co-culturing the Tregs with HLA-A2+PDL1+ K562 cells or mature monocyte-derived HLA-A2+ dendritic cells (DCs) (Figure 2A).
Results: PD1 was efficiently ablated in CAR Tregs with >50% of the CAR+ Tregs expressing tCD19 and having no detectable PD1 protein following expansion. Edited cells maintained their characteristic constitutively high expression of FOXP3 and Helios, indicating a stable Treg product. Upon stimulation with HLA-A2+PDL1+ K562 cells, PD1KOtCD19KI CAR Tregs upregulated activation markers (LAP, GARP, CTLA-4, and 4-1BB) to a greater degree than their PD1-sufficient counterparts, suggesting they are more sensitive to antigen stimulation. They were also significantly more potent than control Tregs at suppressing the expression of co-stimulatory molecules (i.e. CD86) on moDCs upon co-culture, demonstrating an enhanced ability of PD1KOtCD19KI CAR Tregs to suppress the antigen presenting function of DCs (Figure 2B).
Conclusion: PD1 deletion enhances the activation potential and in vitro suppressive function of CAR Tregs. These data suggest that PD1KOtCD19KI CAR Tregs would be more potent as a cell-based therapy to protect allografts. Our future work will assess their function in a humanized mouse model of transplantation in the hopes of further improving the function of genetically modified Tregs.
Juvenile Diabetes Research Foundation. Canadian Institutes of Health Research.
Lectures by Sonya Mangat
| When | Session | Talk Title | Room |
|---|---|---|---|
|
Mon-01 09:20 - 10:20 |
Abstracts Session 2 | CRISPR-mediated deletion of PD1 enhances the function of human CAR Tregs | Grand Georgian |
