Newcastle University
Validation of a novel suspension microcavity system for generation and maintenance of optimally-sized primary human pancreatic pseudo-islets
Morgan Shaw1, Tasnim Ahmed1, Minna Honkanen-Scott1, Markus Mühlemann4, Merilin Georgiou2, Nicole Kattner1, Rowen Coulthard3, James A M Shaw1, Catherine Arden2, Patrick Kugelmeier4, William E Scott III1.
1Translational and Clinical Research Institute, Newcastle University, Newcastle Upon Tyne, United Kingdom; 2Biosciences Institute, Newcastle University, Newcastle Upon Tyne, United Kingdom; 3Department of Cellular Pathology, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle Upon Tyne, United Kingdom; 4Kugelmeiers Ltd., Erlenbach, Austria
Introduction: Rapid loss of viable cell mass over time in culture remains a major limiting factor for human pancreatic islet research and clinical transplantation. Larger islets are particularly vulnerable to hypoxia and necrosis. Impaired clinical transplant outcomes have been reported using deceased donor pancreatic islet isolations with mean diameter >150 µm1. We aimed to evaluate the use of novel 3D microcavity suspension-well plates for rapid generation and maintenance of optimally-sized viable pseudo-islets.
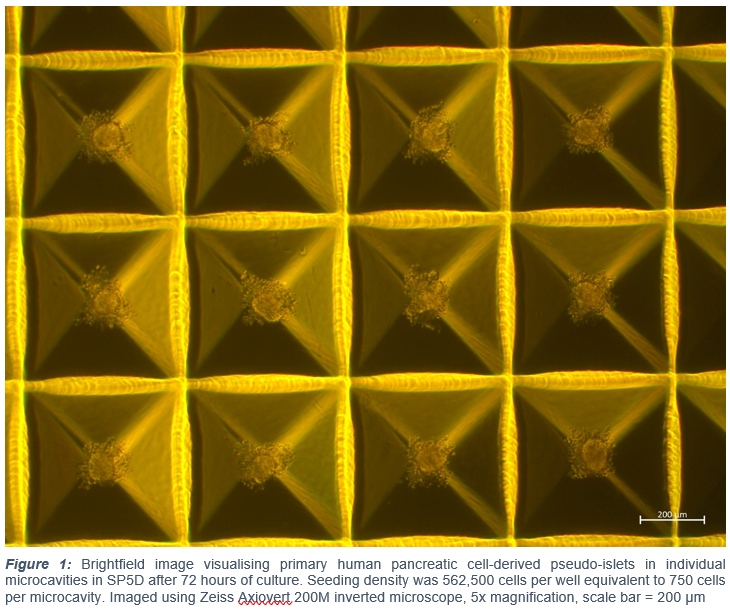
Methods: We utilised a microcavity suspension culture system (SphericalPlate 5D (SP5D), Kugelmeiers Ltd., Erlenbach, Switzerland). Each well contains 750 microcavities. MIN6 murine β-cells were seeded (at densities of 7,500-63,750 cells/well) into SP5D and control 24-well suspension plates (CS) and cultured for 72 hours. Primary human islets were dissociated into single cell suspensions through addition of accutase and mechanical stirring in a 37 °C water bath. Single cells were seeded at densities of 187,500-562,500 cells/well into SP5D plates and cultured for 120 hours. Control human islets were cultured in non-adherent T75 cm3 flasks according to existing protocols. Cells were cultured in 5% CO2 at 37 °C with pseudo-islet diameter assessed using an eyepiece graticule and viability estimated following propidium iodide staining.
Results: In MIN6 cells, mean pseudo-islet diameter of ⁓100 µm after 72 hours’ culture was attained with a seeding density of 52,500 cells/well in SP5D plates (97.2±9.2 µm) vs control cultures (72.5±31.1 µm; n=3 repeated studies (±SD) p<0.02). Comparison of standard deviations demonstrated less size variation in SP5D plates (p=0.003). Accutase-dissociated human islets rapidly formed a single pseudo-islet in each SP5D well (Figure 1). Following 120 hours’ preservation, mean pseudo-islet diameter of ⁓100 µm with no pseudo-islets >150 µm was maintained in SP5D plates seeded with 562,500 cells/well equivalent to 750 cells/microcavity (101.8±7.7 µm; n=50 islets). In non-manipulated control human islets preserved in non-adherent T75 cm3 flasks (total IEQ = 971), mean diameter was significantly higher (132.2±63.1 µm; p=0.001) with largest islets 300 µm in diameter. Viability was maintained throughout the 120 hour preservation period in SP5D plates (88.8±7.9%) and was significantly higher than control (70.2±21.5%; n=50 islets; p<0.0001).
Conclusion: Standardised protocols for preparing pseudo-islets derived from beta-cell line and primary human pancreatic cells in a novel suspension microcavity system have been established. Individual pseudo-islet maintenance per microcavity was achieved with both cell types. Optimal and consistent diameter size of ~100 µm was attained. Viability was maintained in SP5D for up to five days of culture.
Kugelmeiers Ltd.. National Institute for Health and Care Research (NIHR) Blood and Transplant Research Unit (BTRU) in Organ Donation and Transplantation.
References:
[1] Lehmann R, Zuellig RA, Kugelmeier P, Baenninger PB, Moritz W, Perren A, Clavien PA, Weber M, Spinas GA. Superiority of small islets in human islet transplantation. Diabetes. 2007 Mar;56(3):594-603. doi: 10.2337/db06-0779. PMID: 17327426.
Lectures by Morgan F Shaw
| When | Session | Talk Title | Room |
|---|---|---|---|
|
Mon-01 09:20 - 10:20 |
Abstracts Session 2 | Validation of a novel suspension microcavity system for generation and maintenance of optimally-sized primary human pancreatic pseudo-islets | Grand Georgian |
