IgG and IgM ABO antibodies detected by a novel bead-based assay fail to be discriminated by hemagglutination methods
Anne M Halpin1,2,3,4, Tess Ellis3,4,5, Jean Pearcey3,4,5, Bruce Motyka3,4,5, Todd L Lowary4,6,7, Chris Cairo4,6, Stephanie Maier4, Lori J West2,3,4,5.
1Alberta Precision Laboratories, AHS, Edmonton, AB, Canada; 2Laboratory Medicine and Pathology, University of Alberta, Edmonton, AB, Canada; 3Alberta Transplant Institute, University of Alberta, Edmonton, AB, Canada; 4Canadian Donation and Transplantation Research Program, University of Alberta, Edmonton, AB, Canada; 5Pediatrics, University of Alberta, Edmonton, AB, Canada; 6Chemistry, University of Alberta, Edmonton, AB, Canada; 7Academia Sinica, Institute of Biological Chemistry, Taipei, Taiwan
Introduction: ABO antibody (ABO-Ab) quantification is essential for risk assessment in ABO-incompatible (ABOi) transplantation, but the hemagglutination assay (HA) for ABO-Ab titres is known to be plagued by poor reproducibility and variable sensitivity. It is also technically challenging to distinguish IgG vs IgM antibodies by HA methods. Furthermore, red cell HA assays cannot detect ABO-Abs for glycan subtype-specificities; this is a critical factor in ABOi transplantation due to the different expression of ABH subtype glycans on erythrocytes vs tissues/organs, especially endothelium (1,2). We developed a novel bead-based assay to measure subtype-specific ABO-Abs of various isotypes. Our aim was to measure IgG and IgM ABO-Abs as mean fluorescence intensity values and compare to HA titres.
Methods: Healthy adult serum/plasma samples of ABO-A, -B, and -O blood groups (n=119; 60% female) were tested by “standard” HA (no anti-human globulin [AHG]). Serially diluted sera (50uL) were incubated with 25uL of 1% group A1 and group B reagent red cells at room temperature; HA titre was reported as the last dilution showing visual agglutination. The same samples were tested against in-house developed ABO single antigen LuminexTM beads (A and B subtype I-VI glycans coupled to individual beads). ABO-Abs bound to the LuminexTM beads were detected with either anti-human IgG or IgM secondary antibodies.
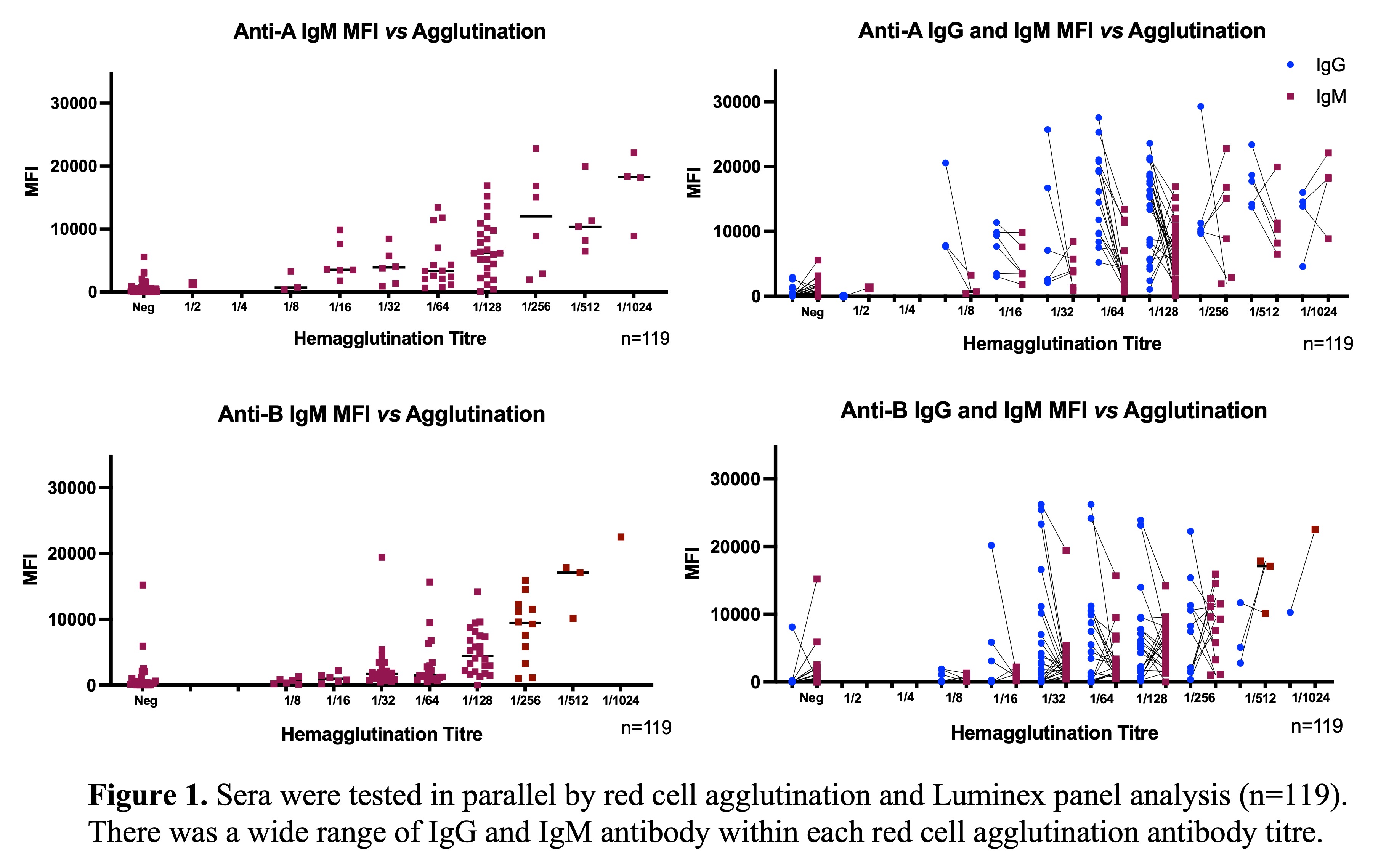
Results: Analyses were focused on ABO-Abs specific to A-II, -III, and -IV and B-II glycan subtypes as these are reported to be the biologically relevant glycan targets. Levels of ABO-Abs with specificities to these A- and B- subtypes were highly variable between individuals and within each ABO HA titre as shown in Figure 1.
A comparison of IgG and IgM isotype antibodies by blood group is shown in Figure 2.
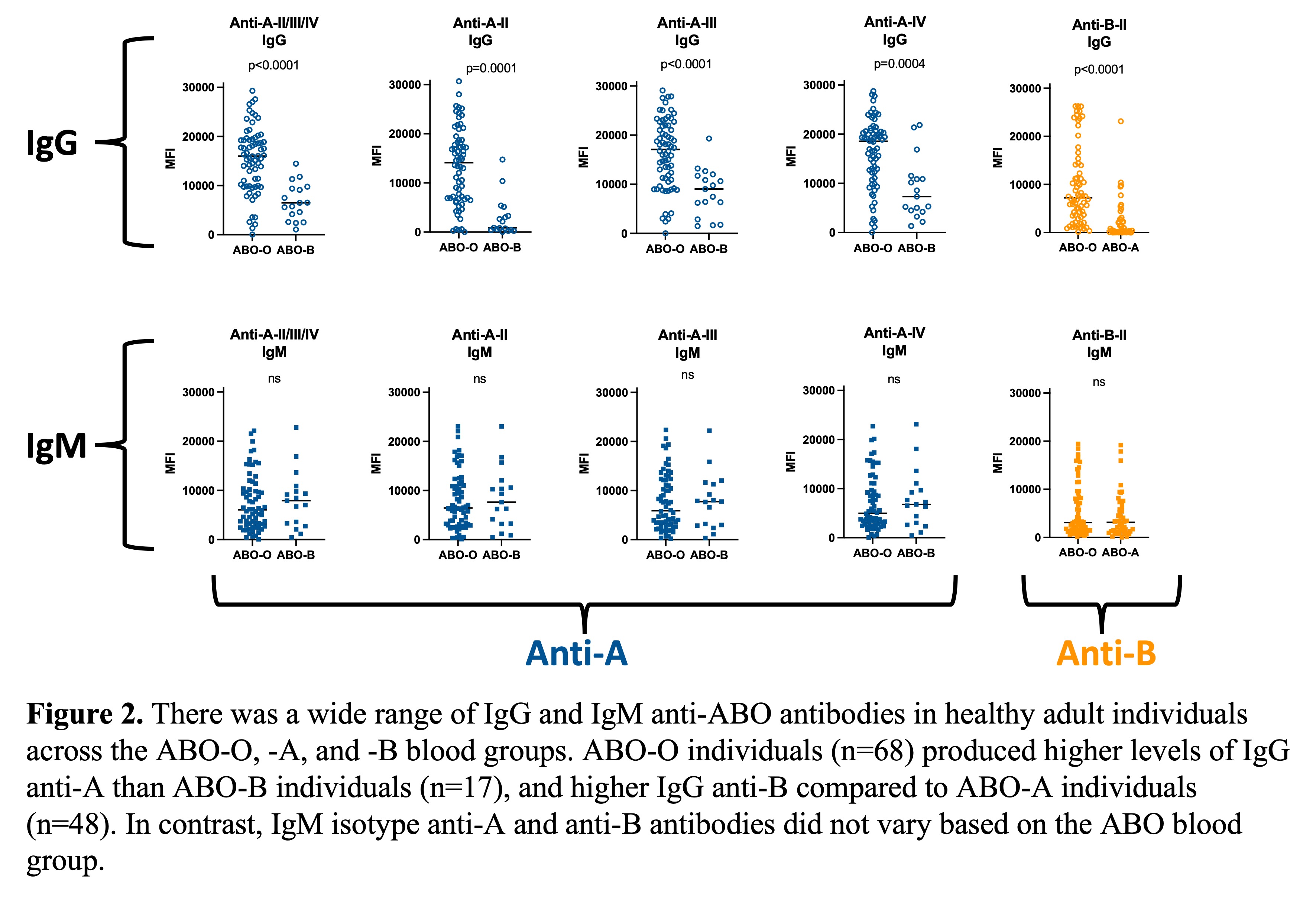
Sera from ABO-O individuals contained significantly higher levels of IgG isotype antibodies to A-II, -III, -IV and to B-II glycans than sera from ABO-B and ABO-A individuals, respectively, but no differences were detected in IgM isotype antibodies amongst sera from individuals of the different ABO blood groups (Figure 2). There were no significant sex-related differences observed (data not shown).
Conclusion: These results demonstrate that HA titre alone is insufficient for accurate assessment of ABOi transplant risk. IgG Abs can contribute to non-AHG HA titres, despite the common assumption that only IgM Abs drive agglutination; each ABO titre includes an unpredictable range of ABO-Abs. This novel ABO-Ab detection method will allow better characterization of the relative roles of IgG vs IgM ABO-Abs in ABOi-transplantation, making it possible to manage immune risk with more isotype-specific tools such as imlifidase. Moreover, the ability to detect Ab subtype-specificities provides organ-specific information that is not possible with erythrocyte agglutination. Tools such as this bead-based ABO assay will enable greater precision in ABOi transplant risk assessment.
1. We gratefully acknowledge support from the Canadian Donation and Transplantation Research Program (CDTRP), GlycoNet, the Women and Children Health Research Institute (WCHRI), and Enduring Hearts/Additional Ventures..
References:
[1] 1. Jeyakanthan M, Meloncelli PJ, Zou L, Lowary TL, Larsen I, Maier S, et al. ABH-Glycan Microarray Characterizes ABO Subtype Antibodies: Fine Specificity of Immune Tolerance After ABO-Incompatible Transplantation. Am J Transplant 2016;16(5):1548–58.
[2] 2. Bentall A, Jeyakanthan M, Braitch M, Cairo CW, Lowary TL, Maier S, et al. Characterization of ABH-subtype donor-specific antibodies in ABO-A-incompatible kidney transplantation. Am J Transplant . 2021;21(11):3649–62.
Lectures by Anne M Halpin
| When | Session | Talk Title | Room |
|---|---|---|---|
|
Sun-30 16:40 - 18:00 |
Abstracts Session 1 | ABO and COVID-19 susceptibility: A role for ABH glycans and ABO antibodies? | Grand Georgian |
|
Mon-01 09:20 - 10:20 |
Abstracts Session 2 | IgG and IgM ABO antibodies detected by a novel bead-based assay fail to be discriminated by hemagglutination methods | Grand Georgian |
