Iron availability alters immune responses after allogenic skin transplantation
Amy Cross1, Joe N Frost2, Marie Sion1, Joanna Hester1, Hal Drakesmith2, Fadi Issa1.
1NDS, University of Oxford, Oxford, United Kingdom; 2MRC Human Immunology Unit, MRC Weatherall Institute of Molecular Medicine, Oxford, United Kingdom
Introduction: Adaptive immune responses demand significant cellular iron availability and usage. Iron supply to immune cells is controlled by the cell surface transferrin receptor and systemically by the hormone, hepcidin. Genetic impairment of cellular iron uptake can present as an immunodeficiency in humans, whilst hypoferremia has been shown to impair responses to vaccination and viral infection. We hypothesized that the manipulation of iron in vivo could control alloimmune responses in a transplantation setting.
Methods: To assess the dependence of T lymphocytes on iron availability for eliciting alloresponses, immunodeficient B6 (H2b) Rag1 knock-out mice received CD4+ T cells from either wild-type mice or TfrcY20H/Y20H mice, where the Y20H substitution decreases the ability of the transferrin receptor to internalize extracellular iron by ~50%. Cell recipients received fully mismatched CBA (H2k) skin, after which transplant survival and the phenotype of peripheral blood immune cells were monitored. To further assess alloresponses in low iron environments, wild-type fully immunocompetent mice received fully mismatched skin transplants and hypoferremia was induced by a low-iron diet and injections of a hepcidin mimetic. Transplant outcomes and the peripheral and intragraft immune compartment were then assessed.
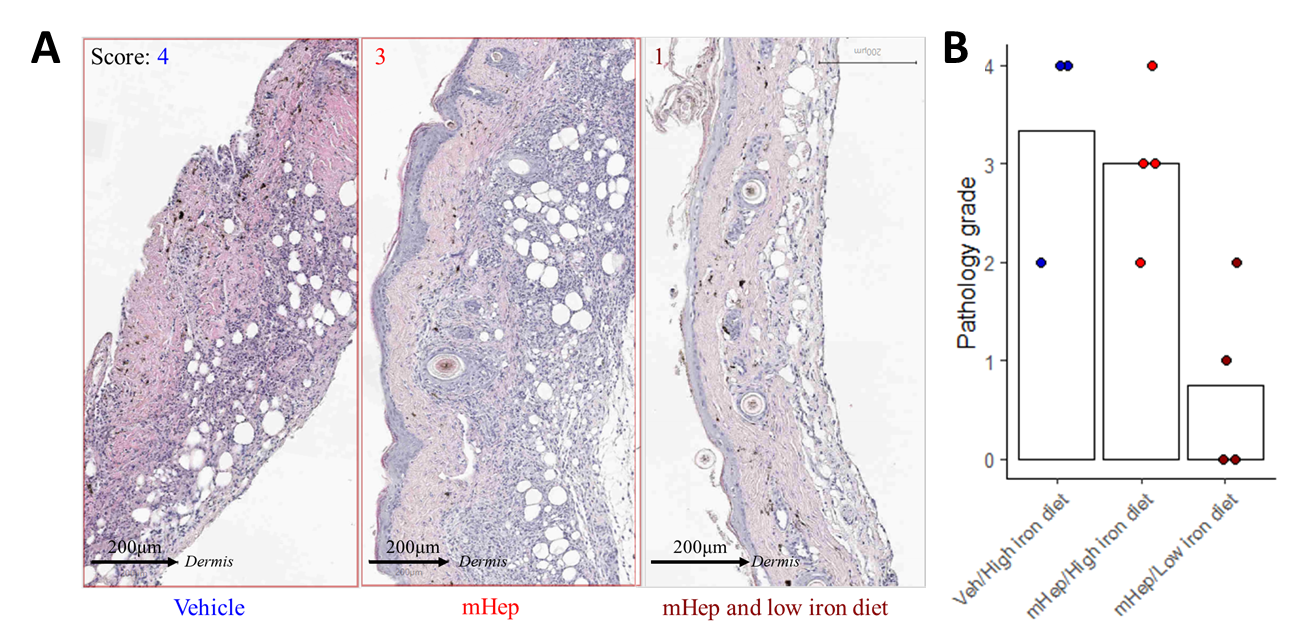
Results: The transfer of wild-type CD4+ T cells into immunodeficient recipients led to transplant rejection with a median survival time of 27 days. Despite maladaptive iron uptake, the TfrcY20H/Y20H T cells were detectable in the blood on days 14 and 21 but were unable to mediate transplant rejection. In a further model of iron insufficiency, systemic hypoferremia suppressed the expansion of alloreactive CD4+ and CD8+ T effectors in the blood and draining lymph nodes of immunocompetent mice that had received fully MHC mismatched skin grafts. Median allograft survival time was prolonged from 9 to 19 days while hepcidin injections continued; however, effector T-cell populations expanded, and the grafts were rejected after treatment was withdrawn. Mice treated with mini-hepcidin alone, without a dietary intervention, also developed hypoferremia with evidence of suppressed effector T cell responses following transplantation. However, mini-hepcidin alone was unable to prevent the accumulation of leukocytes within the allograft by day 8 (Figure 1A-B). This highlights a discordance between peripheral and intragraft responses, wherein the skin was a more permissive environment for alloimmune responses during hypoferremia compared with that of lymphoid organs.
Conclusion: This study demonstrates that manipulating lymphocyte iron availability locally or systemically may control rejection responses and promote transplant survival. Future work will focus on identifying the responsible mechanisms that may be targeted therapeutically without the requirement for systemic hypoferremia.
Lectures by Amy R Cross
| When | Session | Talk Title | Room |
|---|---|---|---|
|
Mon-01 09:20 - 10:20 |
Abstracts Session 2 | Iron availability alters immune responses after allogenic skin transplantation | Grand Georgian |
