Postdoctoral research fellow
Transplant surgery
Brigham and Women's Hospital
A Single Cell Analysis Delineating the Effects of Biological Sex on Alloimmity in Organ Transplantation
Yao Xiao1, Tomohisa Matsunaga1,2, Friederike Martin1,3, Ryoichi Maenosono1,2, Hao Zhou1, Stefan Tullius1.
1Transplant Surgery, Brigham and Women's Hospital, Boston, MA, United States; 2Urology, Osaka Medical and Pharmaceutical University, Osaka, Japan; 3Surgery, Charité - Medical University, Berlin, Germany
Purposes: We have shown clinically that young female recipients have inferior death-censored graft survival independent of donor sex. In contrast, graft survival was superior in older female recipients, suggesting the impact of recipient sex hormones over chromosomal sex mismatches. Experimentally, we have demonstrated that estrogen deprivation dampened Th1 responses while augmenting Tregs systemically. Here, we delineate the impact of estrogen on alloimmunity at a single-cell level.
Methods: Female C57BL/6 (3-month) mice underwent bilateral ovariectomies (OVX) 2 weeks prior to MHC fully-mismatched heterotopic heart transplantation from male DBA/2 donors (n = 5). Allografts were procured at the peak of alloimmune responses (day 5) for morphology and flow analysis. A single cell sequencing with a total of 60,000 sorted CD45+ live cells was performed simultaneously. MLR and DC-T cell assay were performed for ex vivo validation.
Results: Estradiol levels had significantly decreased 2 wks after OVX (2.1 ± 1.1 pg/ml vs. 14.2 ± 7.9 pg/ml in controls), comparable to levels observed in 18 mths old animals (2.5 ± 0.5 pg/ml). OVX significantly prolonged cardiac graft survival (10 vs. 8 days, p < 0.05) with improved morphologies and reduced immune cell infiltration. Graft infiltrates by 5 days revealed decreased antigen-presenting DCs (6.7% vs. 14.1%) and Th1 cells (26.4% vs. 37.6%); amounts of Tregs had increased (18.0% vs. 11.2%; all p < 0.05). ScRNAseq identified distinct and sex hormone-specific cell clusters for monocytes, CD4 and CD8 T cells, B, NK, NKT, neutrophils and DCs. 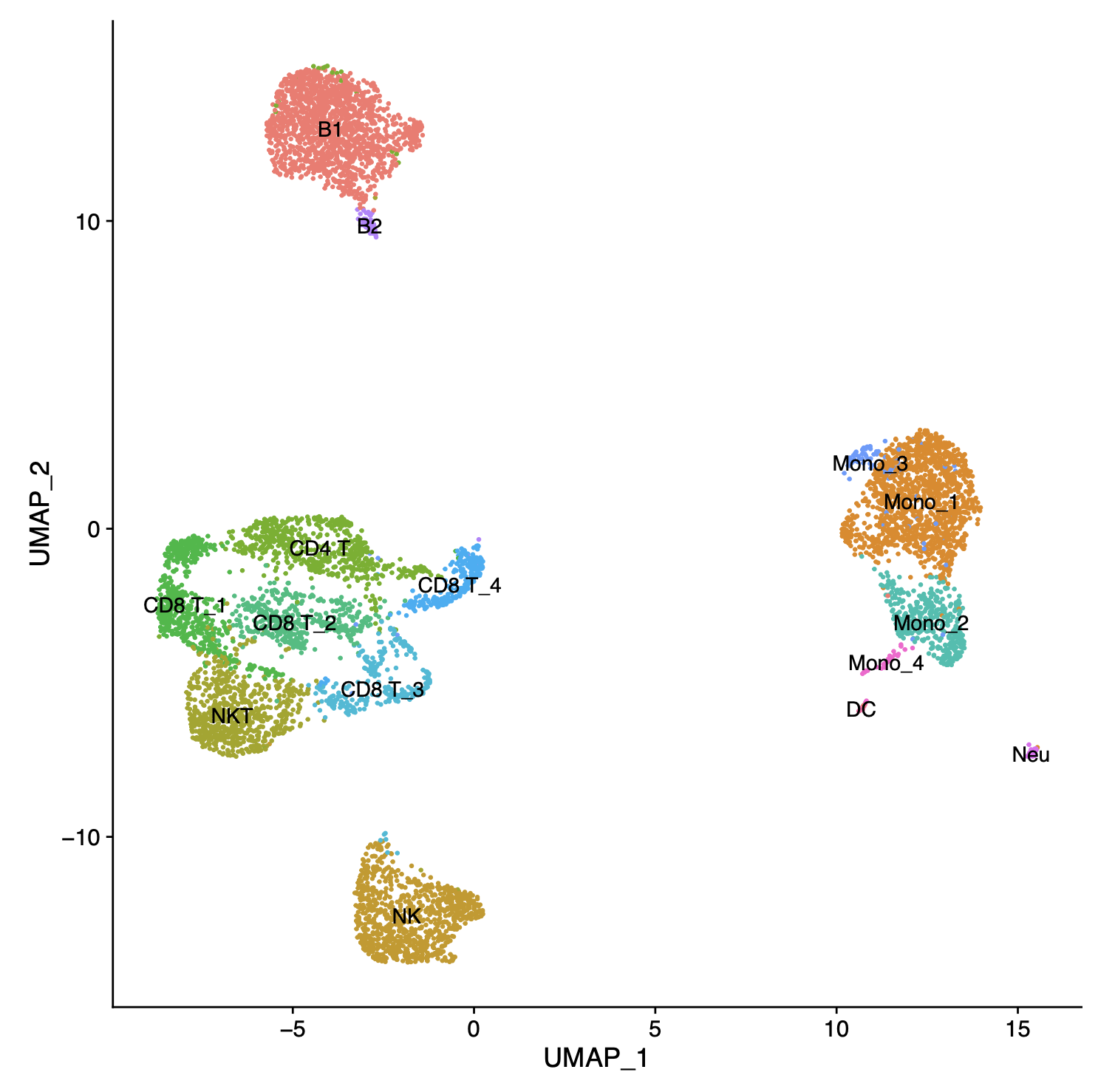 OVX downregulated Tnfsf9, Il2, Il4ra, Cxcr4, Ccl17 and Il15ra expression in Cst3+Ccr7+ DCs, indicating that the activation of cytokine-cytokine receptor and co-stimulation pathways in DCs was inhibited subsequent to estrogen deprivation. A CD4 T cell cluster with a high expression of co-stimulatory (Icos, Cd28, Ctla4) and memory (Il7r, S100a4) cell markers had decreased after ovariectomies, with compromised activation of antigen processing and antigen presentation pathways. Ex vivo coculture of isolated recipient’s DCs with naïve CD4 T cells confirmed the diminished co-stimulatory interactions between DCs and CD4 T cells. In MLR assays, CD4 T cells isolated from OVX mice demonstrated a compromised proliferation and IFN-γ production with increased cell death. Transplants in MHC fully-mismatched CD4-specific ER knockout recipients demonstrated prolonged median graft survivals (p < 0.05) comparable to those observed in OVX recipients, demonstrating a critical role of sex hormones and receptors on alloimmunity.
OVX downregulated Tnfsf9, Il2, Il4ra, Cxcr4, Ccl17 and Il15ra expression in Cst3+Ccr7+ DCs, indicating that the activation of cytokine-cytokine receptor and co-stimulation pathways in DCs was inhibited subsequent to estrogen deprivation. A CD4 T cell cluster with a high expression of co-stimulatory (Icos, Cd28, Ctla4) and memory (Il7r, S100a4) cell markers had decreased after ovariectomies, with compromised activation of antigen processing and antigen presentation pathways. Ex vivo coculture of isolated recipient’s DCs with naïve CD4 T cells confirmed the diminished co-stimulatory interactions between DCs and CD4 T cells. In MLR assays, CD4 T cells isolated from OVX mice demonstrated a compromised proliferation and IFN-γ production with increased cell death. Transplants in MHC fully-mismatched CD4-specific ER knockout recipients demonstrated prolonged median graft survivals (p < 0.05) comparable to those observed in OVX recipients, demonstrating a critical role of sex hormones and receptors on alloimmunity. 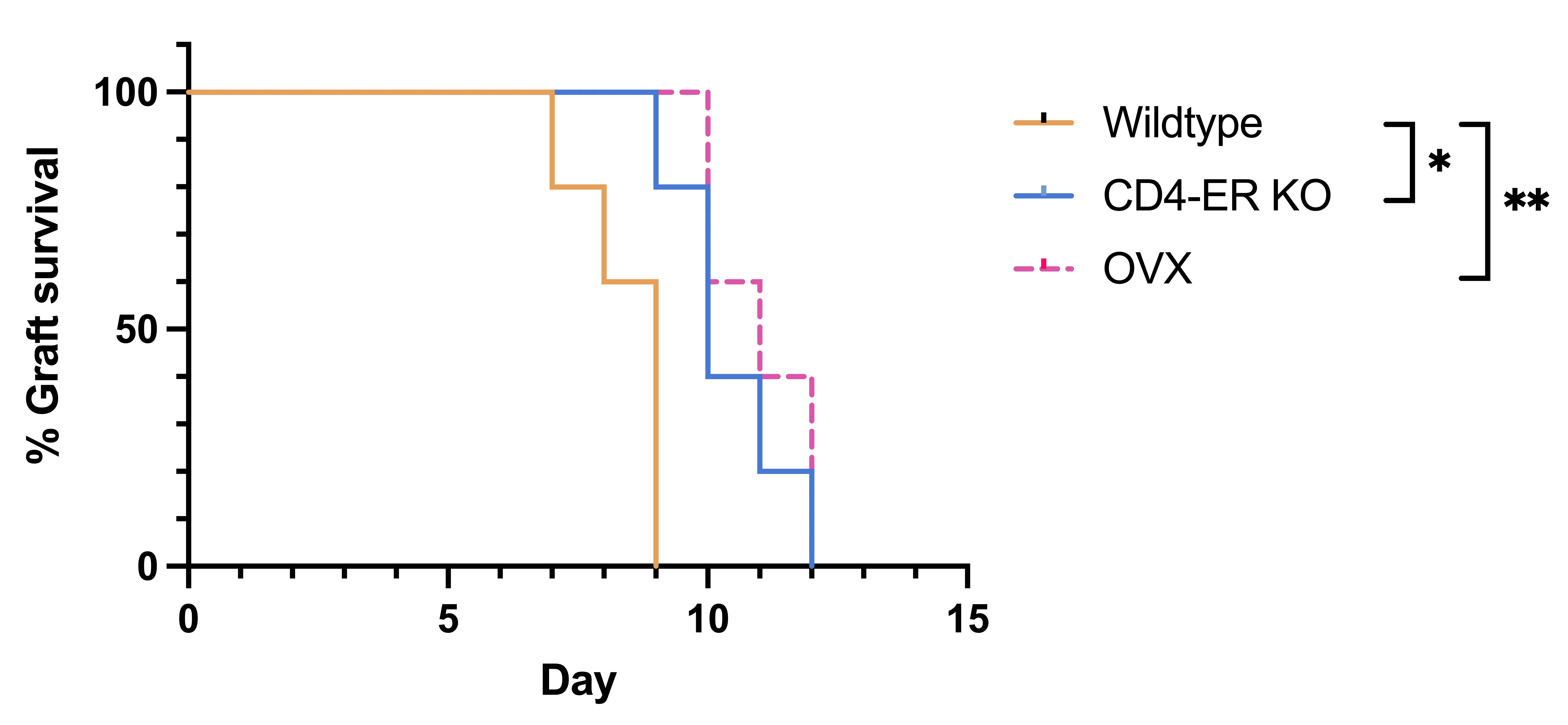
Conclusions: This is the first report of an estrogen-dependent single-cell immune landscape and transcriptomic profile at the peak of alloimmune responses. Those data stress the relevance of biological sex on alloimmunity while providing novel targets allowing sex-specific treatments in and beyond organ transplantation.
S.G.T. is supported by the National Institute of Health (R01AG064165 and U01AI132898). F.M. is suppoted by Sanofi Women in Transplantation Fellowship Award. T.M. is supported by Osaka Medical Foundation Scholarship. .
Lectures by Yao Xiao
| When | Session | Talk Title | Room |
|---|---|---|---|
|
Tue-02 09:20 - 10:30 |
Abstracts Session 3 | A Single Cell Analysis Delineating the Effects of Biological Sex on Alloimmity in Organ Transplantation | Grand Georgian |
