Cardiac allograft monitoring using cardiac magnetic resonance imaging (cMRI) in a porcine heterotopic heart transplant model of acute rejection
Michelle Mendiola Pla1, Carmelo A Milano1, Carolyn Glass2, Dawn E Bowles1, David C Wendell3,4.
1Department of Surgery, Duke University Medical Center, Durham, NC, United States; 2Department of Pathology, Duke University Medical Center, Durham, NC, United States; 3Duke Cardiovascular Magnetic Resonance Center, Duke University Medical Center, Durham, NC, United States; 4Division of Cardiology, Duke University Medical Center, Durham, NC, United States
Introduction: Acute rejection (AR) is a significant complication following cardiac transplantation affecting 24.1% of patients within one-year of transplantation. The primary method for AR monitoring is through histologic assessment of serial endomyocardial biopsies (EMB). This procedure has been performed successfully by our group in a porcine with a heterotopic heart transplant (HHT) in the intra-abdominal position (1), but EMB is limited by sampling error variability. Cardiac magnetic resonance imaging (cMRI) is a non-invasive method for globally monitoring AR in an allograft, but AR progression on cMRI has not been described in this model. Here we use cMRI to characterize changes in tissue parameters of allografts in a porcine HHT model of AR.
Methods: Blood-type matched, swine leukocyte antigen fully mismatched (n=6) Yucatan pig pairs underwent cMRI prior to intra-abdominal HHT. Recipients were treated with tacrolimus, methylprednisolone, and mycophenolate mofetil for 14 days post-transplantation. On post-operative day (POD) 19, the recipients underwent cMRI of the native and transplanted hearts. cMRI studies were comprised of cine imaging, tissue characterization, late-gadolinium enhancement (LGE), and velocity-encoded imaging. Cine imaging was used for LV function and mass. Tissue characterization of myocardial edema used T1 and T2 mapping. LGE imaging investigated the presence of myocardial fibrosis/necrosis. Finally, velocity-encoded imaging was used to verify patent flow to the graft at the anastomotic sites. Troponin values and histologic assessment of EMBs were obtained to concurrently monitor for AR.
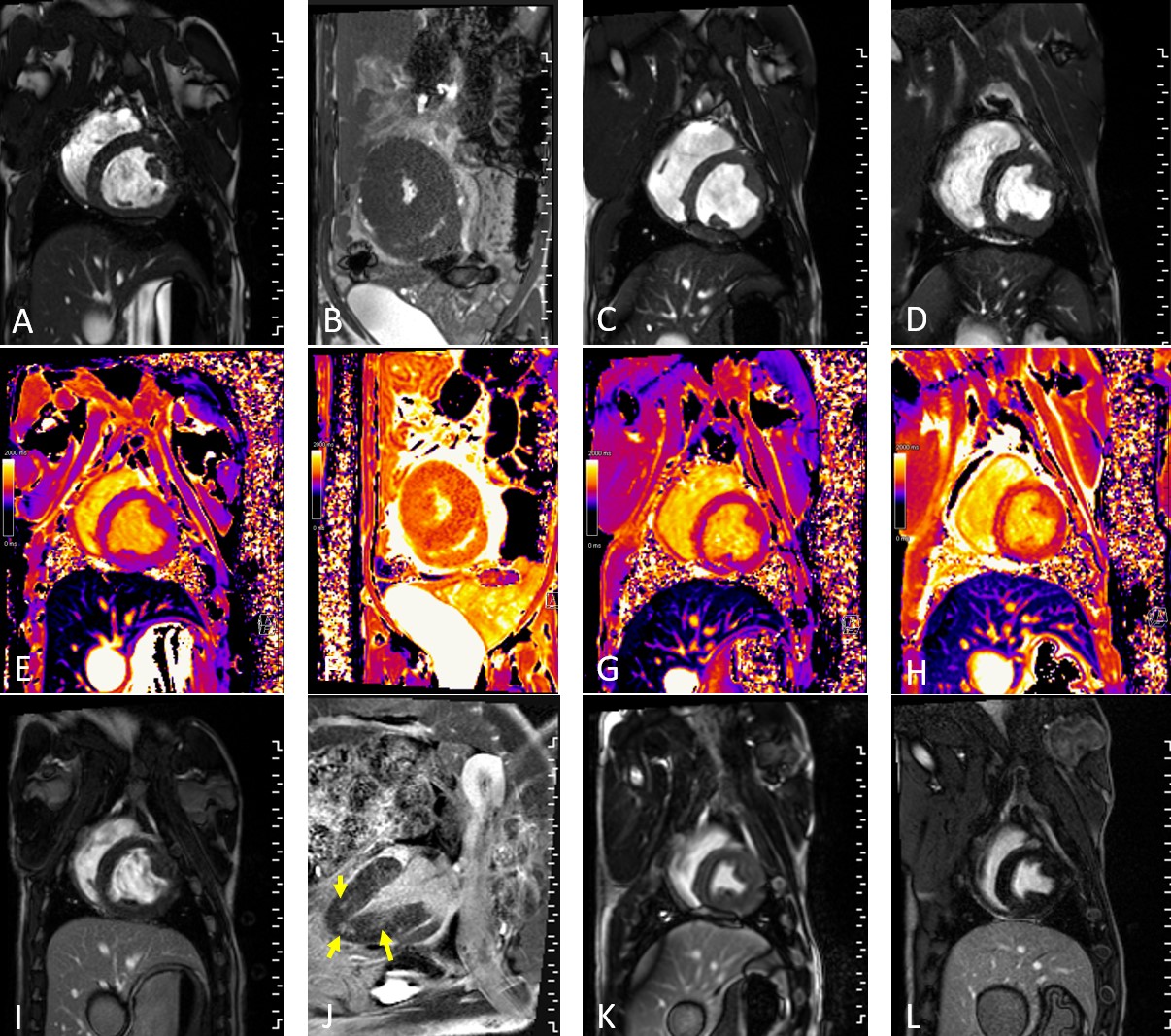
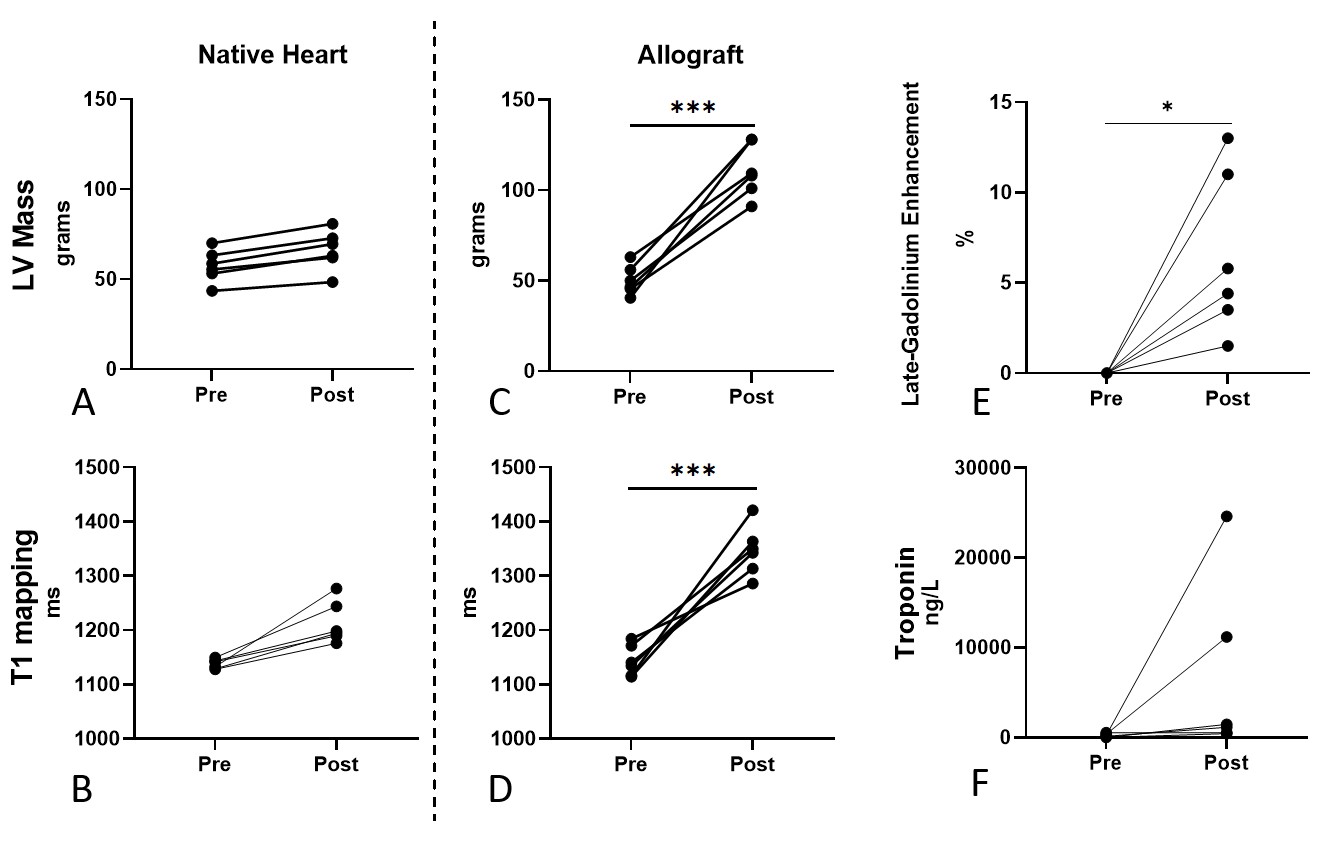
Results: cMRI results are presented as changes from the baseline scan to the POD 19 scan. At baseline, both donor and recipient hearts had normal graft function and no evidence of wall thickening, myocardial inflammation/edema, or presence of hyperenhancement via LGE imaging. There was marked increase in left ventricular (LV) myocardial mass in the allografts (native: 8.7±2.4g increase in LV mass [Figure 1A,B]; allograft: 60.6±16.6g p<0.001 [Figure 1C,D]). T1 mapping values, used to characterize interstitial edema and fibrosis, were also markedly elevated in the allografts (native: 75.7±37.8ms increase in native T1 [Figure1E,F]; allograft: 202.4±69.7ms, p=0.001 [Figure 1G,H]). Contrast imaging demonstrated no fibrosis in native hearts (Figure 1I,J), but increased myocardial fibrosis in all allografts (mean: 6.5±4.5%, p<0.05) in all allografts (Figure 1K-L, Figure 2E). Troponin levels at the time of cMRI were also elevated relative to baseline, indicative of allograft rejection (Figure 2F). All pigs developed histologic severe grade acute cellular rejection with cessation of graft activity at 51±22.4 days.
Conclusion: cMRI demonstrated progression of advanced AR throughout each of the allografts in the intra-abdominal HHT position through increased objective measures of LV mass, interstitial edema, and fibrosis. cMRI is a safe and informative modality for monitoring for AR in a cardiac allograft.
TransMedics, Inc.. Duke Division of Cardiothoracic Surgery.
References:
[1] Mendiola Pla M, Milano CA, Chiang Y, Bishawi M, Kang L, Lee FH, Smith MF, Gross RT, Contreras FJ, Glass C, Bowles DE, Fudim M. Transvenous Endomyocardial Biopsy Technique for Intra-abdominal Heterotopic Cardiac Grafts. J Cardiovasc Transl Res. 2022 Nov 7. doi: 10.1007/s12265-022-10337-7.
Lectures by Michelle Mendiola Pla
| When | Session | Talk Title | Room |
|---|---|---|---|
|
Tue-02 09:20 - 10:30 |
Abstracts Session 3 | Cardiac allograft monitoring using cardiac magnetic resonance imaging (cMRI) in a porcine heterotopic heart transplant model of acute rejection | Grand Georgian |
