Thiago J Borges, United States has been granted the AST-COTS Scientific Congress Award

Immune response of human kidney organoid-based tissues in a humanized mouse model
Thiago Borges1, Yoshikazu Ganchiku1, Ronald van Gaal2, Sebastien G.M. Uzel2, Jeffrey O Aceves2, Kenichi Kobayashi3, Ken Hiratsuka3, Murat Tekguc3, Rodrigo B Gassen1, Ryuji Morizane3, Jennifer A Lewis2, Leonardo V Riella1.
1Center for Transplantation Sciences, Massachusetts General Hospital, Boston, MA, United States; 2Paulson School of Engineering and Applied Sciences and the Wyss Institute, Harvard University, Cambridge, MA, United States; 3Nephrology Division, Massachusetts General Hospital, Boston, MA, United States
Introduction: Given the growing shortage of organs and the substantial morbidity associated with dialysis, there is a need to develop alternative sources of kidney tissue that would replicate a normal kidney function. Recently, differentiation protocols have been developed to generate “mini-kidneys” from human-induced pluripotent stem cells. These organoids exhibit remarkable tissue microarchitectures with high cellular density akin to their in vivo counterparts. Integrative approaches that combine scalable organoid differentiation with tissue biomanufacturing as well as the evaluation of immune responses and host integration are needed to bridge the gap from organoid building blocks (OBBs) to therapeutic organs. The aim of this study is to create human kidney tissues assembled from organoid building blocks and assess their immune responses and host vascular integration in a humanized mouse model.
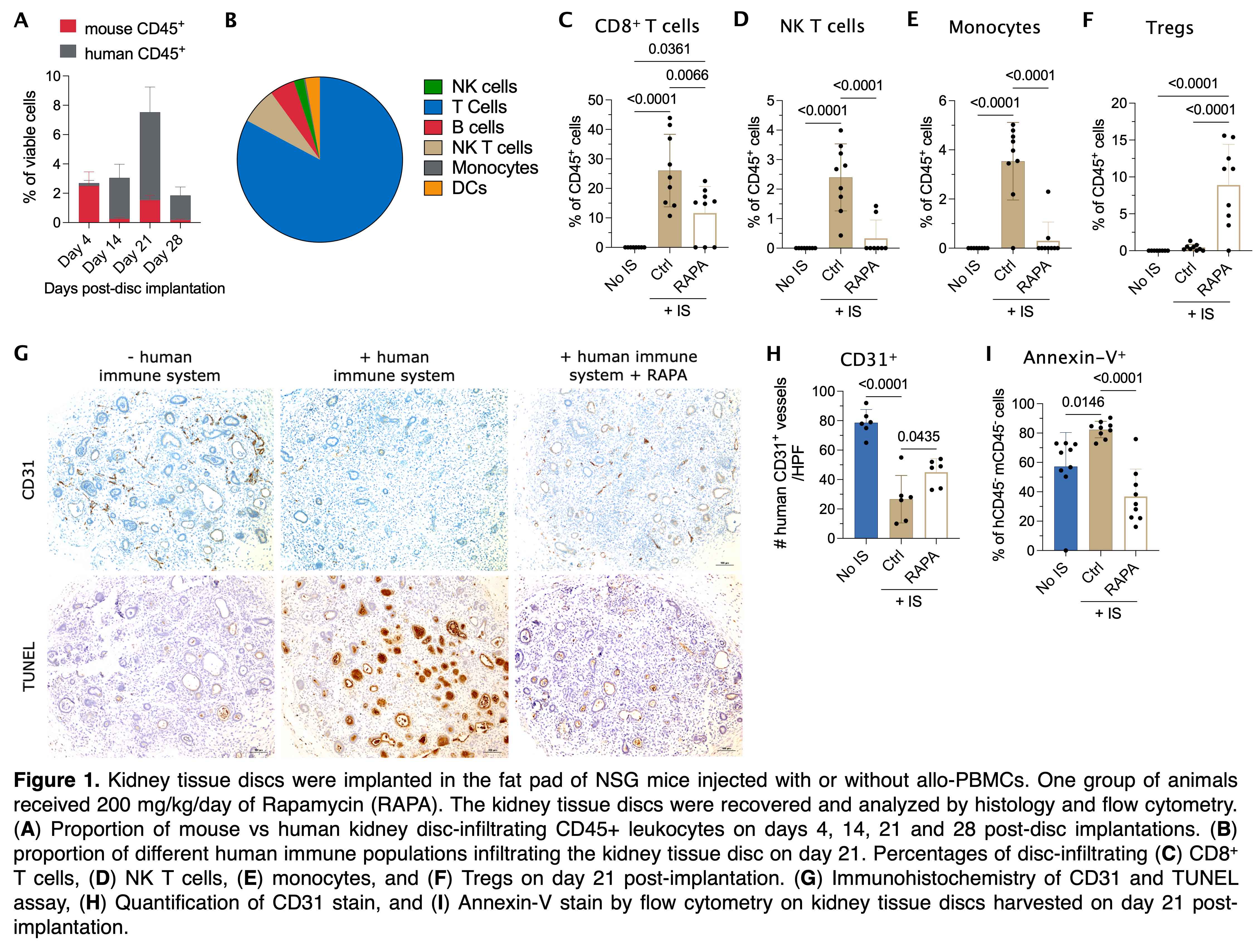
Methods: Kidney organoids are differentiated from the BJFF human induced pluripotent stem cell line. Next, the organoids are suspended in a fibrinogen solution, consolidated, and molded into thin discs (~10 mm3). The kidney tissue discs are then implanted in the fat pad of female NSG mice injected with or without allo-PBMCs. Both BJFF cells and PBMCs were HLA typed and presented 4 HLA mismatches (A1, B2 and DR1). One cohort of animals received 200 mg/kg/day of Rapamycin (Rapa). The kidney tissue discs are recovered on days 4, 14, 21 and 28 post-disc implantations and analyzed by histology and flow cytometry.
Results: Our data demonstrated that the peak of the immune response in the kidney discs was at day 21 post-PBMC injection (Fig 1A). The majority of human CD45+ leukocytes were composed of T cells (~82%) followed by NK T cells (~7%, Fig 1B). Disc-infiltrating CD8+ T cells (Fig 1C), NK T cells (Fig 1D), and monocytes (Fig 1E) proportionally increased over time, which was abrogated by the treatment with Rapa. As expected, Rapa treatment also increased the proportion of Tregs in the discs (Fig 1F). In the presence of an immune system, the kidney organoids had less vascularization (CD31 IHC stain) which was improved by the Rapa treatment (Fig 1G-H). Apoptosis of kidney cells in the discs was significantly increased in discs harvested from PBMC-injected animals by either TUNEL (Fig 1G) or Annexin-V (Fig 1I) stains. This enhanced apoptosis was diminished in animals treated with Rapa (Fig 1G, I).
Conclusion: Our data indicate the success of generating viable mini-kidney discs, the feasibility of the analysis of immune responses against the implanted kidney tissues, and some of the challenges of transplanting these discs into HLA-incompatible recipients.